Abstract
Objective
In the majority of animal experiments, vein puncture is necessary for the drugs administration. This study aimed to propose a new vein puncture method, ultrasound-guided internal jugular vein (IJV) puncture, and compare it with the traditional tail vein puncture.
Methods
We divided 24 male SpragueDawley rats randomly into 2 groups: 12 rats in the tail vein puncture group and other 12 rats in the ultrasound-guided IJV puncture group. After successful puncture, rats from two groups were injected with 0.1 mL ultrasound contrast agents. The average puncture time, the success rate of the first puncture, and the imaging effects of contrast-enhanced ultrasound in the sciatic nerve and liver parenchyma of rats after injecting ultrasound contrast agents were evaluated using time–intensity curves for both different puncture methods.
Results
The average puncture time of the ultrasound-guided IJV group was lower than that of the tail vein puncture group ( p = 0.013), and the success rate of the first puncture was significantly higher than that of the tail vein puncture group ( p = 0.037). There were no significant differences in the imaging effects of contrast-enhanced ultrasound on the sciatic nerve and liver parenchyma between the two different puncture methods. Additionally, neither of the two puncture methods resulted in obvious symptoms such as hematoma formation, convulsions, restlessness or even death in rats.
Conclusions
Ultrasound-guided IJV puncture could be a safe, effective method with a high success rate for rat vein puncture and drug administration, which could be an alternative to rat tail vein puncture.
Introduction
Rodents are used frequently as subjects in basic experimental research for fundamental studies, due to their small size, rapid growth and reproduction, affordability and ease of management. In some experiments, drug administration is required. The administration routes include intramuscular injection, intraperitoneal injection, intravenous injection, and so on [ ]. Among them, intravenous injection has higher bioavailability and faster absorption rate [ ]. Tail vein injection is the most common intravenous administration route for rats and mice [ ]. However, the extremely thin diameter and fragility of the tail vein increase the difficulty of tail vein puncture [ ]. Especially for rats, the position of their tail veins is deeper than that of mice, resulting in a lower one-time puncture success rate. In addition, repeated punctures into the same vein become increasingly difficult, due to vessel stenosis caused by fibrotic changes [ ]. Once the puncture fails, it may result in the inability to drug administration, thereby affecting the experimental effect. The internal jugular vein (IJV) is the largest venous trunk in the neck, and the IJV is one of commonly used puncture route for administration in clinical practice [ , ]. However, because the IJV is located in the neck and its peripheral structure is complex, the direct percutaneous puncture of the IJV in rats has been reported rarely. With the widespread application of ultrasound technology, visual puncture is becoming more common. Therefore, ultrasound-guided IJV puncture may be also feasible in rats.
Therefore, to find an alternative to tail vein injection, this study aimed to explore the feasibility of ultrasound-guided IJV injection in rats, and evaluate the imaging effects of contrast-enhanced ultrasound (CEUS) after injecting ultrasound contrast agent.
Materials and methods
Ethical statement
The study protocol was approved by the Animal Experimental Ethics Committee of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. All animal experiments were carried out in strict accordance with the governmental and international animal experiment guidelines (certificate number: SIAT-IRB-150213-YGS-ZHR-A0094-2). Every effort was made to minimize suffering.
Experimental animals and experimental groups
Male Sprague–Dawley rats (8 weeks old, weighing 220–250 g) were purchased from Zhuhai BesTest Biotechnology Co., Ltd (BesTest, Zhuhai, China). They were housed individually in a temperature-controlled (25 ± 2°C) and humidity-controlled (55 ± 5%) facility with a 12 h light:dark cycle. Each rat had free access to food and water. Before puncture, rats were anesthetized with 2% isoflurane at a flow rate of 2.0 L/min. A total of 24 rats were divided into 2 groups: 12 rats in the tail vein puncture group and 12 rats in the ultrasound-guided IJV puncture group. In each group, six rats were used to observe the contrast-enhanced characteristics of the sciatic nerves, and another six rats in each group were used to observe the contrast-enhanced characteristics of the livers. All vein puncture procedures were performed by the same operator.
Tail vein puncture
For the tail vein puncture, after the rat was anesthetized, its tail was secured and wiped with a 75% alcohol cotton ball to dilate the rat’s tail vein before injection, and then a 26G indwelling needle was used for tail vein puncture ( Fig. 1 ). When inserting the needle, the operator used the index finger and thumb of the left hand to secure the rat’s tail, allowing it to bend downward after passing through the thumb. Once the tail vein was clearly visible, the operator gently inserted the needle into tail vein at an angle of approximately 30°, with the needle tip facing upward. After inserted into the tail vein, the needle immediately became parallel to the tail vein. Then the needle was gently withdrawn to observe obvious backflow, which indicated a successful puncture. The injection process should proceed without any obvious resistance under normal circumstances, and the tail vein should not bulge. When pushing the liquid, gentle movements should be required. If a bulging tail vein was noticed, it indicated that the needle had not pierced into the tail vein and needed to be withdrawn immediately and reinserted. After successfully puncturing, the core of the intravenous indwelling needle was removed, and its tube was retained in the tail vein and was secured with tape.

Ultrasound-guided IJV puncture
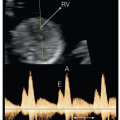
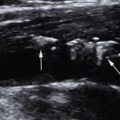
For ultrasound-guided IJV puncture, a Mindray ultrasound imaging device (Resona 7, Shenzhen, China) equipped with a 4 to 15 MHz linear-array probe (L11-3U) was used for ultrasound guidance. The probe was wrapped with a sterile plastic sleeve. The rats were placed in a supine and left lateral position, fully exposing the neck, chest, upper abdomen, and right thigh. The B-mode ultrasound imaging and/or color Doppler flow imaging were used to display the right IJV. The direction of the probe was adjusted to place the target IJV in the center of the screen to confirm the best puncture point and safe puncture path ( Fig. 2 a, b). The right hand held the probe, and the left hand held a 26G venous indwelling needle. The vein indwelling needle was slowly inserted into IJV at an angle of approximately 30° to 45° to the skin under the real-time ultrasound guidance. Two puncture methods can be used according to the different positions of the ultrasound probe and the needle insertion point: a long axis plane and short axis plane. The specific choice of the best puncture method and path can be based on the operator’s habits. The short axis plane puncture method was used in this study. Ultrasound scanning was used to track the position of the needle tip to ensure that the venous indwelling needle was placed into the IJV correctly. A 1 mL syringe was connected to the venous indwelling needle. When the syringe was slowly withdrawn, the dark red venous blood was drawn, indicating a successful puncture ( Fig. 2 c). After successfully puncture, the needle core of the indwelling needle was pulled out, and the needle tube was detained in the IJV and was secured with tape ( Fig. 2 d).

CEUS procedure and evaluation
To evaluate the effectiveness of two intravenous administration routes, we used injection of ultrasound contrast agent for CEUS examination, so that the administration effect can be displayed easily and intuitively. The enhancement effects of superficial tissues (such as the sciatic nerve) and internal organs (such as the liver) were evaluated and compared. The same ultrasound equipment (Resona 7) and linear-array transducer (L11-3U) was used. Before CEUS examination, the target tissue or organ should be displayed clearly on B-mode ultrasound imaging. The image parameters (including gain, frequency, focus, etc.) were optimized and fixed. The ultrasound probe was fixed in a constant position using a device that could clamp and immobilize the probe during the following CEUS examination. SonoVue (Bracco Imaging, Milan, Italy) was used as ultrasound contrast agent in this study. After shift to CEUS mode, a bolus of SonoVue (0.1 mL/rat) was injected intravenously through the tail vein or IJV, followed by a 3.0 mL saline to flush. The whole CEUS examination was stored as a video of at least 180 s on the ultrasound machine for subsequent analysis. The region of interest was positioned on the distal segments of the sciatic nerve and left lobe of the liver, and then the time–intensity curve and related parameters for microvascular perfusion in the nerve tissue and liver parenchyma were automatically generated. The area under the curve (AUC) was used to reflect the relative blood volume of the tissue, the peak intensity (PI) was used to reflect the maximum signal intensity during one bolus injection and the time to peak (TTP) corresponded with the time interval from the beginning of enhancement to the maximal enhancement. These parameters were used for a comprehensive evaluation of the CEUS effect of two different intravenous injection methods.
Data analysis
Comparison was made between the average puncture time and the success rate of the first puncture for the tail vein puncture group and ultrasound-guided IJV puncture group. The average puncture time referred to the duration from locating the vein to placing the needle inside the vein successfully. The success rate of the first puncture referred to the ratio of the number of rats that were punctured successfully on the first puncture to the total number of rats that were punctured successfully.
Safety evaluation of different vein puncture methods
At the end of the experiment, each rat was observed to closely evaluate the safety of the two vein puncture routes, mainly to observe the occurrence of hematoma formation, animal convulsions, mania and even death.
Statistical analysis
GraphPad Prism 9.4 (GraphPad, Software Inc., La Jolla, CA) was used for statistical analysis and plotting, and all data were represented as mean ± standard error of the mean. Count data were expressed as numbers (%) and were compared using Fisher’s exact test. To determine statistical significance, the differences between two groups were analyzed by the Student t -test. A p value of less than 0.05 was considered statistically significant.
Results
Comparison of different vein puncture methods
The results of this experiment are presented in Table 1 . As indicated in Table 1 , the average puncture time for tail vein was 9.79 ± 0.38 min, the success rate for first puncture was 58.33% (7/12) and all other rats were punctured successfully after repeated attempts. The average puncture time of the IJV under ultrasound guidance was 8.46 ± 0.31 min, and the success rate for first puncture was 100% (12/12). These results indicated that the average puncture time for the tail vein was significantly longer than that for ultrasound-guided IJV puncture ( p < 0.05). This difference may be attributed to the higher first puncture success rate for ultrasound-guided IJV puncture group compared with the tail vein puncture group ( p < 0.05), thus avoiding prolonged puncture times caused by repeated attempts.