Introduction
Patients with aortic pathology often present with nonspecific complaints or presentations such as back pain, abdominal pain, myocardial infarction (MI), or even stroke, making the diagnosis difficult. The adage of “abdominal pain plus one other organ system” should prompt the physician to consider aortic pathology as the unifying diagnosis. The level and acuity of pain often is secondary to the etiology of the pain. For example, chronic pain may be secondary to an expanding aortic aneurysm, whereas more acute pain may be secondary to aortic dissection or traumatic aortic rupture. Abnormalities of the liver, spleen, and colon should also be considered in the differential listed in Box 20.1 .
- •
Renal: Nephrolithiasis, UTI, severe hydronephrosis
- •
GI: Diverticulitis, inflammatory bowel disease, SBO, cholecystitis, pancreatitis, hepatitis, splenic infarct
- •
Compression of surrounding structures due to mass lesion
Normal Anatomy
The aorta originates from the left ventricle of the heart and ascends, giving off branches to supply blood to the upper body and brain. The ascending aorta courses left and caudally, forming the aortic arch and the descending aorta. The aorta tapers as it descends from the thoracic into the abdominal cavity, ultimately bifurcating into the common iliac arteries ( Fig. 20.1 ).
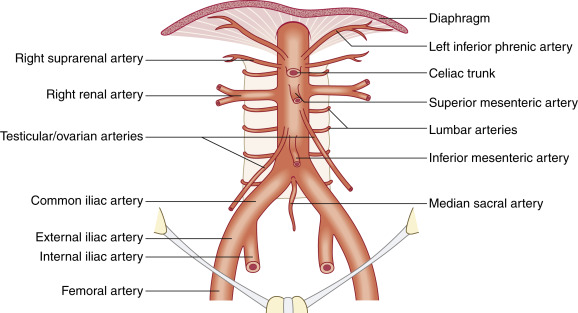
The first and most proximal branch of the abdominal aorta is the celiac axis. It divides into three main branches: the splenic, common hepatic, and left gastric arteries. The left branch becomes the splenic artery, and the right branch the common hepatic artery. There is a smaller division of the celiac axis, the left gastric artery, which is often not well visualized on ultrasound.
The second large branch of the abdominal aorta, approximately 1 to 2 cm caudal but sometimes abutting the celiac axis, is the superior mesenteric artery (SMA), which lies anterior to the aorta. The splenic vein passes over the SMA, emptying into the portal vein just right of midline. The left renal vein passes between the SMA and the aorta before emptying into the inferior vena cava (IVC).
Sonographic Findings
Imaging of the aorta is normally performed using a curved array transducer, typically 3 MHz frequencies, depending on body habitus. The probe indicator should face the patient’s head for coronal and sagittal views or the anatomic right side for axial views. It is important to image the entirety of the abdominal aorta in both long and short axis, starting superiorly in the epigastric region and scanning through the proximal common iliac vessels. As the aorta descends, it follows the lordosis of the spine, allowing for more superficial ultrasound imaging.
It is best to start the examination by identifying posterior landmarks such as the vertebral body, a rounded, hyperechoic structure with posterior shadowing. Depth is typically set in the 20- to 30-cm range to identify these structures and set the far field, depending on abdominal girth. Once the vertebral body is identified, the depth can be adjusted to visualize the structures of interest, keeping the vertebral body visible ( Fig. 20.2 , ![]() ).
).
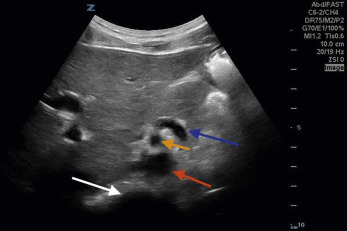
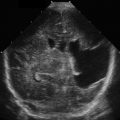
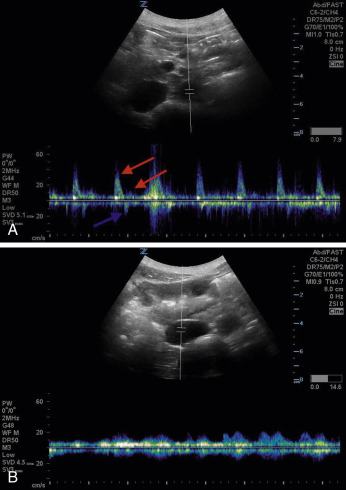
The aorta will rest anterior and to the anatomic left of the vertebral body. On the right, the IVC runs alongside the aorta; thus the two structures must be sonographically distinguished from one another. The IVC is usually respirophasic, thin walled, and collapsible, depending on patient’s hydration status and phase of respiration. The aorta is thicker walled, non-collapsing, and pulsatile. When pulse wave Doppler is placed on an aorta with normal flow, it has a triphasic appearance ( Fig. 20.3A ) compared with the IVC, which has a monophasic waveform of differing morphology, depending on the phase of respiration (see Fig. 20.3B ).
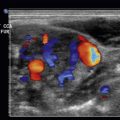
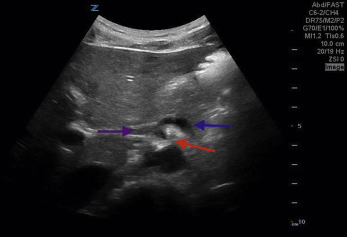
The major branches of the aorta often have characteristic sonographic appearances. The axial view of the celiac axis originating from the aorta is commonly known as a seagull sign ( Fig. 20.4 , ![]() ). The axial view of the SMA takeoff surrounded with hyperechoic fatty tissue is known as the Mantel clock sign . The pancreas and the liver are tissues anterior to the splenic vein that can be seen at the level of the SMA ( Fig. 20.5 ,
). The axial view of the SMA takeoff surrounded with hyperechoic fatty tissue is known as the Mantel clock sign . The pancreas and the liver are tissues anterior to the splenic vein that can be seen at the level of the SMA ( Fig. 20.5 , ![]() ).
).
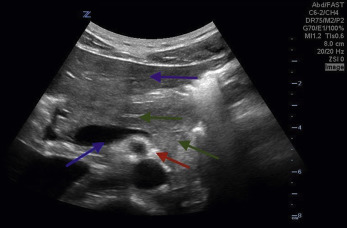
Several centimeters inferior to the takeoff of the SMA, the origin of the right and left renal arteries are seen ( Fig. 20.6 , ![]() ). The abdominal aorta ultimately bifurcates into the right and left common iliac arteries ( Fig. 20.7 ,
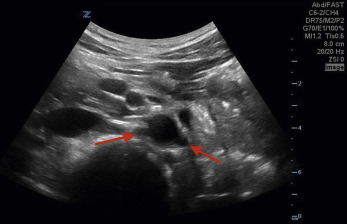
). The abdominal aorta ultimately bifurcates into the right and left common iliac arteries ( Fig. 20.7 , ![]() ).
).
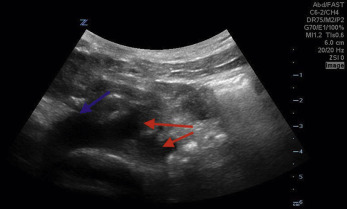
Ultrasound Technique and Tips
Visualization of the abdominal aorta can be challenging, depending on the patient’s body habitus, bowel contents, and specific anatomy. The patient should be supine, and it may be advantageous to have the patient bend their knees with the feet on the bed. This rocks the pelvis posteriorly, allowing the rectus abdominal muscles to relax, giving the operator improved ability for bowel compression.
To visualize the proximal aorta, the liver can be utilized as an acoustic window. Asking the patient to take a deep breath lowers the diaphragm and liver, creating a larger acoustic window. If the proximal aorta is not readily visualized in the upper abdomen, the patient can be rolled to the left lateral decubitus position to displace bowel gas. If still having difficulty with visualization, the probe can be placed in the right midaxillary line to utilize the right lobe of the liver as an acoustic window. In this view, the aorta will appear in the far field, with the IVC in the near field. Alternatively, the abdominal aorta may be imaged left parasagittally, where the rectus abdominis meets the oblique abdominal musculature. Angling the probe toward the spine in this position helps undercut anterior gaseous distention of the bowel.
If the patient’s abdominal girth prohibits acceptable image acquisition, using the lowest-frequency probe on the lowest-frequency setting can help penetrance and improve visualization. If bowel gas proves a challenge, use graded compression with the ultrasound probe. Applying gentle, steady compression can dissipate bowel gas by physically displacing the intestine in the mid-to-lower abdomen. Bowel gas in the upper abdomen is more difficult to avoid, as the proximal duodenum is tethered by its retroperitoneal segments and is not easily moved.
Pathology
Abdominal Aortic Aneurysm
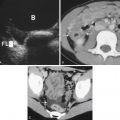
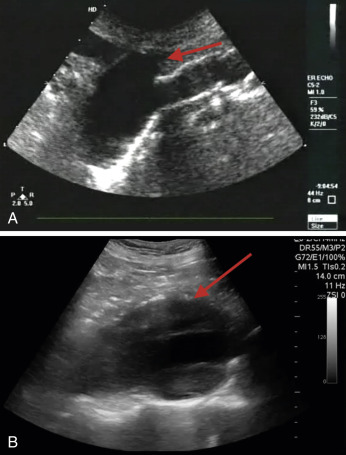
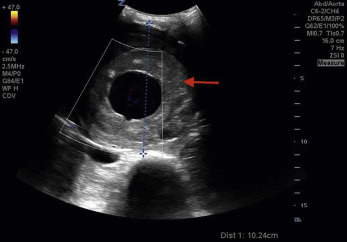
An abdominal aortic aneurysm (AAA) occurs when there is a focal enlargement of the abdominal aorta >3 cm or >50% enlargement of the normal diameter. AAAs are usually asymptomatic until they are large or rupture. Risk factors for AAA are shown in Table 20.1 . A transverse view of the aorta will identify both saccular—a focal outpouching of the aortic wall ( Fig. 20.8A , ![]() )—and fusiform—concentrically dilated—aneurysms, as shown in Fig. 20.8B , and
)—and fusiform—concentrically dilated—aneurysms, as shown in Fig. 20.8B , and ![]() . Measurement of the AAA should occur in the short-axis view to avoid the cylinder tangent error of a long-axis view, with the calipers placed on the anterior outer wall to the posterior outer wall ( Fig. 20.9 ). Ninety percent of AAAs occur below the level of the renal arteries.
. Measurement of the AAA should occur in the short-axis view to avoid the cylinder tangent error of a long-axis view, with the calipers placed on the anterior outer wall to the posterior outer wall ( Fig. 20.9 ). Ninety percent of AAAs occur below the level of the renal arteries.
| Age |
| Male gender |
| Smoking |
| Hypertension |
| Hyperlipidemia |
| Inherited genetic defects (Marfan’s syndrome, Ehlers-Danlos syndrome, Turner’s syndrome) |
| Intramural hematoma |
| Penetrating atherosclerotic ulcer |
| Atherosclerotic disease |
| Inflammatory and infectious aortitis along with an underlying aortic dissection |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree