Abdominal Arteries, Venous System, and Nonvascular Intervention
Michael J. Miller Jr.
Tony P. Smith
Abdominal Aorta and Its Branches
Abdominal Aortography and Intervention
Although individualized to a particular patient and their clinical situation, angiography of the abdominal aorta is most often performed for atherosclerotic disease, both aneurysmal and occlusive. Angiography is also performed for aortic dissection and trauma. Rarely, involvement with the vasculitides including Takayasu arteritis, mid-aortic syndrome, and other etiologies necessitate angiography.
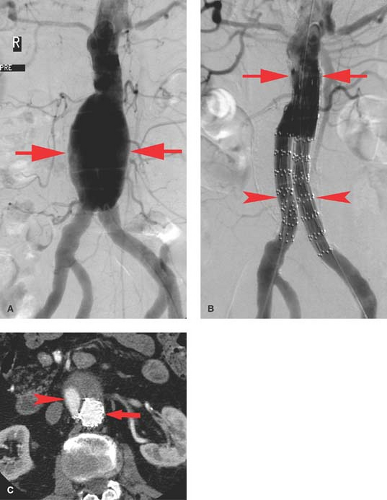
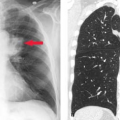
Aneurysms. As with the thoracic aorta, there are multiple possible etiologies for abdominal aortic aneurysm (AAA). The two of primary importance are atherosclerosis which we will refer to as AAA and infection which we will refer to as mycotic. The most common etiology of AAAs is atherosclerosis. AAA is defined as enlargement of the aorta 1.5 times greater than the normal vessel diameter. For the most part, they are fusiform and often lined with mural thrombus. Although US can demonstrate the aneurysm and be used for screening and surveillance, CT has become the diagnostic study of choice. Angiography demonstrates only the true lumen, not the portion of the aneurysm, which is thrombus filled. This results in underestimation of the aneurysm diameter. This limitation, the advancement of CT angiography, and the potential risks associated with angiography have resulted in a reduction in its use. Angiographically, AAA is seen as an irregular, often calcified, fusiform aneurysm (Fig. 24.1A). Angiography can evaluate the patency of other major vessels (renals, visceral, and iliacs) and their relationship to the aneurysm.
Aneurysm screening is recommended for men with a history of smoking and over the age of 65. If there is a family history, screening should be started at 60. There is a higher incidence of aneurysm in males, Caucasian race, and smokers. The annual risk of aneurysm rupture increases with increasing diameter (Table 24.1). Aneurysms are felt to cause 15,000 deaths annually in the United States. Treatment is recommended for aneurysms which are greater than 50 mm. AAA expansion of greater than 5 mm in a 6-month period raises the concern for rupture and is also a recommendation for treatment.
It is common for AAA to extend into the iliac arteries, and 99% of atherosclerotic iliac artery aneurysms are associated with an AAA. Treatment of AAA had been traditionally by open surgical repair. However, stent graft placement has become widely accepted due to its minimal invasiveness (Fig. 24.1). A number of stent grafts are available requiring access sites via the common femoral artery for placement and therefore are most often placed using surgical access to one or both groins. The grafts differ by design including segments without covering which can be anchored above the renal arteries as well as bifurcated sections for the iliac arteries. Stent grafts can be successfully placed into AAA in over 90% of cases. Approximately 25% will require additional endovascular procedures. The decision to treat an aneurysm using endovascular exclusion typically depends upon the diameter and length of the neck, presence of angulation, and the diameter of the common and external iliacs in addition to the presence of internal iliac aneurysms. The neck is defined as the normal portion of the aorta between the lowest renal artery and the beginning of the aneurysm. Large-diameter or short-length necks or severe angulation increases the risk for failing to exclude the aneurysm and the probability of endoleak.
To that end, one of the major concerns with the placement of an endograft is the continued filling (opacification) of the AAA following stent graft placement, termed endoleaks. Such leaks are best studied with CT (Fig. 24.1C) as well as by angiography particularly as endovascular techniques are utilized to repair such leaks. Endoleaks are categorized into four types: Type 1 is a leak at the superior or inferior attachment site, Type 2 represents AAA filling via a patient arterial side branch
such as a lumbar or the inferior mesenteric artery (IMA), Type 3 is the loss of integrity of the stent graft, and Type 4 is the leak through the porous graft material. Isolated common iliac artery atherosclerotic aneurysms can be handled much like AAAs using smaller stent grafts. Internal iliac artery atherosclerotic aneurysms are probably best managed by embolizing the internal iliac aneurysm primarily with coils.
such as a lumbar or the inferior mesenteric artery (IMA), Type 3 is the loss of integrity of the stent graft, and Type 4 is the leak through the porous graft material. Isolated common iliac artery atherosclerotic aneurysms can be handled much like AAAs using smaller stent grafts. Internal iliac artery atherosclerotic aneurysms are probably best managed by embolizing the internal iliac aneurysm primarily with coils.
Table 24.1 Aortic Diameter-Related Risk of Rupture | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mycotic aneurysms or psuedoaneurysms of the abdominal aorta are a rare but life-threatening condition and are often surgical emergencies. Radiographically, they often appear saccular and very irregular. Their rate of growth and the patient’s constitutional symptoms suggest the infectious nature. The commonest pathogen is Salmonella species, accounting for an incidence of up to 74%.
Aortoiliac occlusive disease is most commonly caused by atherosclerosis. Patients with aortoiliac disease usually present with claudication. The most dramatic example is termed Leriche syndrome which is the combination of bilateral buttock claudication, impotence, and absent femoral pulses. This is typically associated with the occlusion of the infrarenal abdominal aorta. Radiographically, atherosclerotic involvement of the distal aorta and iliac arteries is identical to elsewhere with plaque formation, calcification, and vessel narrowing. Visualization of the aorta and iliac arteries as well as the entire lower extremity can be carried out with CT or MR as well as by angiography. In the setting of absent femoral pulses, the use of CT or MR angiography can help with
determining the extent of disease and help with procedural planning.
determining the extent of disease and help with procedural planning.
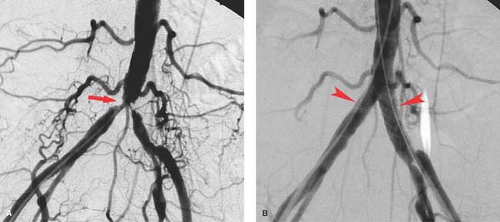
Aortoiliac occlusive disease is probably best initially approached with endovascular techniques including angioplasty with or without stent placement (Fig. 24.2). Following angiographic assessment and prior to treatment of aortoiliac lesions, pressure measurements may be obtained, and a gradient of 10 mm Hg systolic is considered significant. If no gradient is noted, pressures can be augmented with vasodilators injected intra-arterially down the leg to simulate increased blood flow to chemically mimic exercise. Another pressure gradient is then obtained again looking for a difference of 10 mm Hg or higher. Indications for angioplasty should take into account the patient’s symptoms, appearance of the lesion, and pressure gradients. Cases where there is absence of a gradient the lesion may be a source of distal emboli which can result in blue toe syndrome.
Isolated aortic lesions are quite rare but can be effectively treated by endovascular techniques. Disease affecting both the aorta and proximal iliac arteries is usually treated with “kissing” balloons at the aortic bifurcation placed from each common femoral artery. The balloons are sized to fit the iliac arteries and together at the top to the aorta. Success can readily be achieved even with complete occlusion of one or both iliac arteries. Stent placement for aortic or iliac disease is based on the success of angioplasty as determined radiographically, by follow-up pressure gradients or by intravascular sonography. Any residual gradient, vessel irregularity, or intimal flap is an indication for stent placement, although many interventionists stent these vessels primarily instead of even attempting angioplasty alone. Complications of angioplasty are the same as angioplasty at other sites and include acute thrombosis, distal embolization, and vessel perforation which occur all combined in less than 5% of cases.
Aortoiliac occlusive disease can occur from inflammatory diseases, in particular Takayasu arteritis, which produces a long-segment, smooth narrowing of the abdominal aorta which may extend into the branch vessels. Hypoplastic aortic syndrome is a congenital process of unknown etiology producing long-segment narrowing of the aorta usually seen in young females. Neurofibromatosis may also involve the aorta, and the iliac arteries are the third most common location (following the renal and carotid arteries) for fibromuscular disease (FMD).
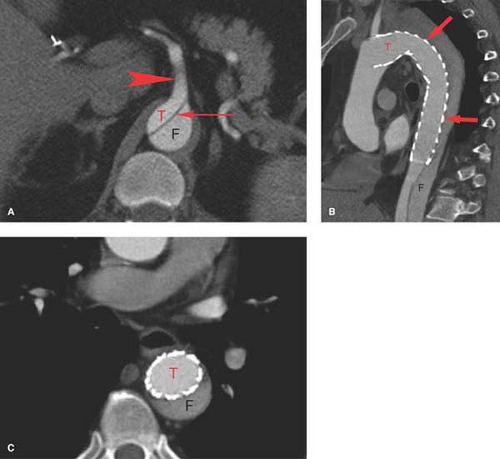
Abdominal aortic dissection is almost always associated with, or in fact is an extension of, thoracic dissection. Abdominal aortic dissections are best imaged by CT, although MR does provide excellent diagnostic images. CT demonstrates both lumens, how well they fill with contrast material, the proximal and distal extent of the dissection, as well as the patency and relationship of branch vessels to the dissection. Besides rupture, of greatest importance is how the dissection plane affects the abdominal aortic branch vessels including visceral, renal, and aortic bifurcation. Angiography is usually reserved for symptomatic patients prior to intervention. Like other imaging, it is essential that angiography demonstrate the extent of the dissection and the patency of branch vessels (Fig. 24.3).
The emergence of a number of endovascular techniques has significantly impacted the management of patients with aortic dissection. Endovascular techniques include stent grafting for the entry site (the origin of the dissection), closure to prevent aneurysmal widening of the false lumen, balloon fenestration of the intimal flap, and aortic true lumen stenting to alleviate branch vessel ischemia. Aortic fenestration is a technique whereby a re-entry needle is introduced via the groin access. Using intravascular ultrasound (IVUS), a puncture is made from one lumen to the other. Following placement of a guide wire, a balloon is used to create an opening between the two aortic lumens to equalize the pressure between the two lumens, providing a reasonable flow into branch vessels from either the true or false lumen. Aortic fenestration is a procedure reserved for emergency situations. False lumen thrombosis following the entry closure with stent grafts has been observed in 86% to 100% of patients, whereas percutaneous interventions are able to effectively relieve organ ischemia in approximately 90% of the cases. As devices and techniques progress, it is to be expected that endovascular techniques will become the method of choice for treating most complications of type-B dissections.
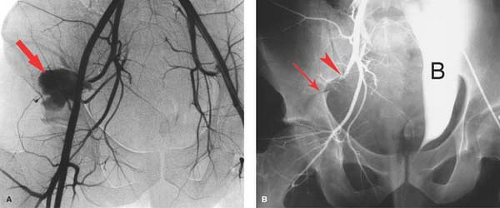
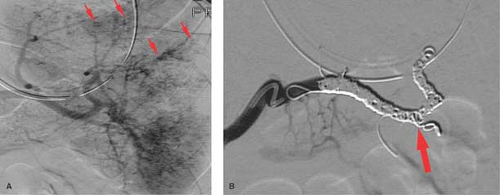
Trauma. Patients rarely survive abdominal aortic trauma, but pelvic trauma resulting in significant bleeding is more commonly seen. Bleeding associated with pelvic fractures may be controlled by fixation devices for those who cannot routinely come to angiography for imaging and embolotherapy. Arterial bleeding is most often associated with injury to one or more branches of the internal iliac arteries and is often demonstrated on CT during trauma evaluation. Embolization is often performed with proximal agents such as gelatin sponge if a temporary agent is desired or coils for more permanent occlusion. Bleeding associated with pelvic fractures can be effectively controlled with embolotherapy (Fig. 24.4). However, repeat angiography should be performed in patients with pelvic fractures with ongoing evidence of hemorrhage demonstrated by persistent base deficit and hypotension once other potential sources of bleeding have been excluded.
Renal Angiography and Intervention
A single renal artery occurs approximately 60% of the time, with either multiple arteries or an early renal artery division in the other 40%. The most common reason to study the renal arteries in the United States is for occlusive disease and trauma, less often for other abnormalities such as neoplasms.
Imaging of the renal arteries includes US, CT, and MR, with nuclear scintigraphy also playing a role in the relative perfusion of the kidney. US provides visualization of the proximal renal artery and evaluates flow. CT and MR angiography has continued to improve and are useful diagnostic tools when renal artery stenosis is clinically suspected. Renal angiography remains the gold standard once screening suggests an abnormality. Angiography not only provides diagnostic images, but endovascular therapy can be applied in the same sitting and is often the primary goal for the angiographic procedure.
Renal arteries are often studied in patients with decreased renal function. In such patients, alternative imaging not requiring iodinated contrast is recommended but when angiography is required there are several useful options. Alternative contrast agents such as carbon dioxide have been successfully used, particularly in conjunction with small doses of iodinated contrast. Alternatively, traditional low or iso-osmolar iodinated contrast material may be used and kept to a minimum especially if the patient has received preventative therapy; chief among these is hydration which is currently the only proven renal protective maneuver.
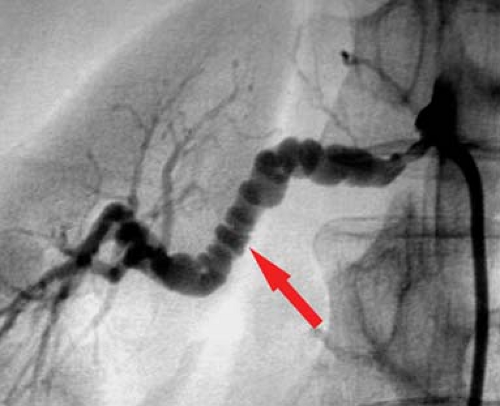
There are a number of etiologies for renal artery occlusive disease including dissection, vasospasm, vasculitis, coarctation syndromes, and neurofibromatosis, but the two most common causes by far are atherosclerosis and FMD. These two account for 99% of the stenoses encountered in the United States, with atherosclerosis representing 65% overall (Fig. 24.5).
Fibromuscular disease (FMD) is the most common cause of renovascular hypertension in patents younger than 40 years and represents an assortment of histological patterns usually classified into three main types based on the morphological
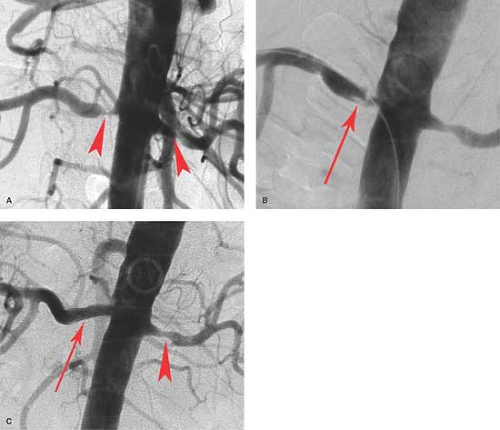
appearance and the layer of the involved arterial wall: intimal (7% to 8%), medial (85%), and periarterial (7% to 8%), although there are also schemes that include subclassifications of each category. Medial FMD accounts for the majority of cases and has the classic “string of beads” appearance on angiography, which represents alternating web-like stenoses and aneurysms (Fig. 24.6). The middle and distal main renal artery is most frequently involved. The proximal renal artery is rarely involved alone. FMD is the most common cause of hypertension in children. It has been shown that atherosclerotic renal disease is progressive, but it is also well documented that FMD can be progressive in nature. FMD classically responds well to angioplasty alone, with success rates approaching 98%. Stenting is rarely required unless there is a dissection resulting from angioplasty.
appearance and the layer of the involved arterial wall: intimal (7% to 8%), medial (85%), and periarterial (7% to 8%), although there are also schemes that include subclassifications of each category. Medial FMD accounts for the majority of cases and has the classic “string of beads” appearance on angiography, which represents alternating web-like stenoses and aneurysms (Fig. 24.6). The middle and distal main renal artery is most frequently involved. The proximal renal artery is rarely involved alone. FMD is the most common cause of hypertension in children. It has been shown that atherosclerotic renal disease is progressive, but it is also well documented that FMD can be progressive in nature. FMD classically responds well to angioplasty alone, with success rates approaching 98%. Stenting is rarely required unless there is a dissection resulting from angioplasty.
Atherosclerotic renal occlusive disease clinically presents with hypertension, renal failure, or both. Patients with atherosclerotic renal disease are usually over the age of 60. The lesions appear like atherosclerosis elsewhere (Fig. 24.5). Atherosclerotic renal artery stenosis is amenable to angioplasty but is not as straightforward as simply dilating the lesion. Anatomically, the atherosclerotic renal artery lesion is in the proximal renal artery and the aorta, the latter is rarely responsive to angioplasty alone. Stenting of renal atherosclerotic lesions for the most part overcomes this and is the standard, but despite an anatomically successful procedure, the patient may not respond very well. In fact, greater than 95% of patients have essential hypertension, and the etiology of renal insufficiency is often multifactorial. Whether treatment of a renal artery stenosis will actually produce a desired clinical result is difficult to predict. Even pressure gradients across the stenosis are not very predictive. Therefore, one can see that the results are never going to be extremely high as one never really knows if the disease process (hypertension and/or renal failure) is actually caused by the renal artery stenosis until treatment is undertaken. To that end, the results of renal angioplasty are often reported as cured (of all antihypertensive medications), improved (requiring significantly less medication), or failed (no change or worsened by treatment). Based on meta-analysis data, with a mean of almost 2-year follow-up, approximately 20% of patients are cured of hypertension with stenting, compared to only 10% following percutaneous angioplasty alone. Improvement of hypertension is about the same with angioplasty alone (53%) versus stent placement (49%). However, an improvement in renal function is better with angioplasty alone (38%) versus stent placement (30%). Restenosis rates are however better with stent placement (17%) when compared to angioplasty alone (26%).
Finally, as with any invasive procedure, there can be complications in 5% to 10% of cases; chief among those is the worsening renal function and injury to the renal artery. In spite of the difficulties with results and the possible complications from angioplasty, endovascular therapy remains the best available treatment following medical failure. The reason to image the renal arteries in a patient with hypertension and/or renal failure is to look for a treatable cause, and a large portion of that treatment is angioplasty and stenting.
Neurofibromatosis causes renal artery stenosis by extrinsic compression of the renal artery by neurofibromata or from disorganized intimal and medial proliferation at the renal artery orifice or in the proximal renal artery. Angiography demonstrates smooth or nodular stenoses with or without associated aneurysms. Hypertension secondary to neurofibromatosis is seen mainly in children.
Renal transplant artery narrowing is most often due to surgical technical factors (acutely) or intimal hyperplasia at the anastomotic site (late) or even from atherosclerosis (much later), all of which may be amenable to balloon angioplasty with or without stenting.
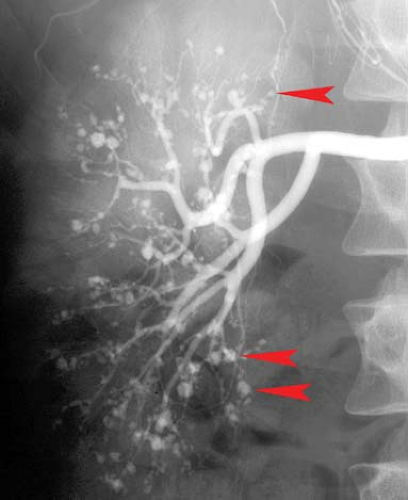
Renal artery aneurysms, exclusive of trauma, are rare (<0.1%). Aneurysms are of two types, extrarenal due to atherosclerosis or FMD and multiple small aneurysms within the kidney associated with polyarteritis nodosa (PAN). For unruptured hilar aneurysms, treatment is indicated when the aneurysm exceeds 2 cm in size and is often amenable to endovascular therapy. PAN is a rare necrotizing vasculitis which affects the small- and medium-sized arteries of multiple organs, most commonly the renal (85%) and hepatic (65%) arteries. Characteristic subcutaneous nodules are seen in 15%. The major angiographic findings are multiple, small, saccular microaneurysms, occlusions, and irregular stenoses throughout the abdominal viscera (Fig. 24.7). Microaneurysms are seen in 50% of patients, ranging in size from 1 to 12 mm and are typically located at branch points. The differential diagnosis of the microaneurysms includes PAN, Wegener granulomatosis, systemic lupus erythematosus, rheumat oid vasculitis, and drug abuse.
Angiography for renal neoplasms is rare; however it may be indicated for preoperative embolization prior to surgical resection or palliative for hematuria. Renal cell carcinoma and angiomyolipoma can be hypervascular, but definitive diagnosis cannot be made angiographically.
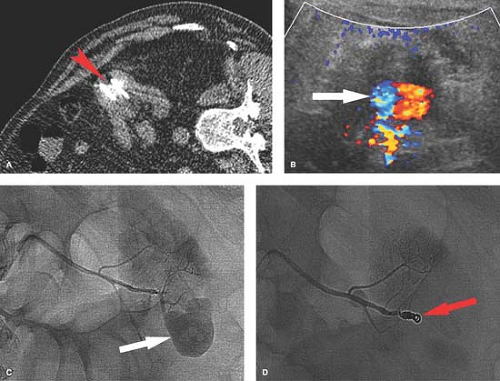
Trauma. Injury to the kidney occurs with penetrating or major blunt trauma. A full range of injuries can be encountered from hematuria without visible injury to a shattered kidney or renal hilum avulsion. Angiography is most often performed in these patients to confirm the extent of injury or to perform endovascular treatment of the injury if possible (Table 24.2). Iatrogenic trauma to the kidney from biopsy or catheter placement can occur (Fig. 24.8). Renal transplant may undergo repeated biopsies. Angiography can determine the site and nature of the injury which include pseudoaneurysm, arteriovenous fistula (AVF), or even extravasation. Most of these injury sites are amenable to endovascular treatment most often by subselective catheterization and coiling.
Splenic Angiography and Intervention
The splenic artery arises from the celiac artery at the hepatolienogastric trunk. In 25% left gastric, splenic, and hepatic
arteries arise as a tripod celiac. Infrequently, the splenic may arise from the aorta or the superior mesenteric artery (SMA). The splenic artery tends to become increasingly tortuous with age. Common branches off the splenic artery include the dorsal pancreatic, arteria pancreatica magna, caudal pancreatic, left gastroepiploic, short gastric, and splenic polar branches.
arteries arise as a tripod celiac. Infrequently, the splenic may arise from the aorta or the superior mesenteric artery (SMA). The splenic artery tends to become increasingly tortuous with age. Common branches off the splenic artery include the dorsal pancreatic, arteria pancreatica magna, caudal pancreatic, left gastroepiploic, short gastric, and splenic polar branches.
There are a few indications for splenic arteriogram and intervention. Complications of any organ embolization also apply to the spleen including dissection or vascular injury. Specific to the spleen are pancreatitis and splenic infarction with abscess formation. Patients should be given pneumococcal vaccine prior to the procedure when time allows.
Trauma is a common indication for splenic arteriogram and endovascular intervention in the hemodynamically stable patient. If the patient is unstable, surgical splenectomy is performed emergently. The risk of delayed rupture increases with the severity of splenic injury. When acute extravasation or a vascular abnormality such as pseudoaneurysm or fistula is discovered, subselective arteriography and embolization of the branch involved are performed. Coils are preferred for embolization of the main splenic artery or its branches. In the setting of severe splenic trauma without extravasation, proximal occlusion of the splenic artery can be performed to preserve splenic tissue through collateral supply from pancreatic and short gastric collaterals while reducing splenic arterial pressure and subsequent decrease in bleeding (Fig. 24.9).
Delayed complications include rebleeding and abscess and may mandate splenectomy.
Hypersplenism is associated with hemolytic anemia, splenic vein thrombosis, portal venous hypertension, tumor, infiltrative diseases, myelofibrosis, and polycythemia vera. Anemia, thrombocytopenia, splenomegaly causing discomfort, and gastric varices due to splenic vein thrombosis are indications for partial splenic infarction. Embolization is performed with a distal agent such as gelatin sponge, 355 to 500 micron polyvinyl alcohol sponge particles, or 500 to 700 micron calibrated gelatin microspheres until 60% to 70% of the splenic tissue is ablated. It is imperative the catheter be distal to pancreatic branches prior to infusion of the embolic agent to prevent pancreatitis. Splenic abscess is a complication of splenic embolization and may result in the patient needing splenectomy.
Table 24.2 American Association for Surgery of Trauma Renal Trauma Grading Scale | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
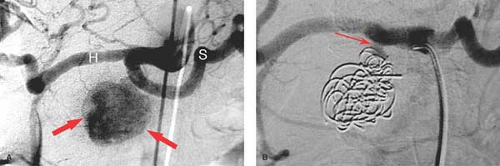
Splenic artery aneurysm is the most common aneurysm outside of the aorta and iliac arteries. They are encountered between the third and sixth decade of life and are more common in females. Congenital aneurysm has a higher rate of rupture. The diagnosis is typically made with CT or MR. Aneurysms and pseudoaneurysm can be treated with coil obliteration, trapping of the arterial segment with coils, or detachable plugs (Fig. 24.10). Blood flow to the spleen is usually preserved through short gastric collaterals. Multiple aneurysms of the main and branch vessels of the splenic artery can be seen in the setting of cirrhosis with resultant portal hypertension.
Hepatic Angiography and Intervention
The common hepatic artery arises from the celiac trunk. The gastroduodenal artery (GDA) is its first major branch, with the artery continuing as the proper hepatic artery. Branch arteries parallel the portal veins and supply the liver segments. In approximately 55% of people, the right, left, and middle hepatic artery arises from the common hepatic artery. The cystic artery often arises from the right posterior branch. The middle hepatic artery may arise from either the left or the right hepatic artery. It supplies liver segments IVa and IVb.
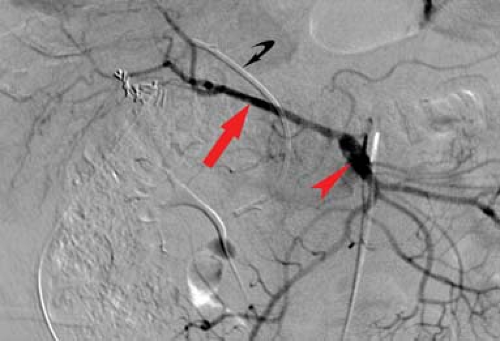
In approximately 2.5% of people, the common hepatic artery arises from the superior mesenteric artery (SMA). An aberrant right hepatic artery exists in up to 26% of people. The most common variations are either a replaced right hepatic artery (Fig. 24.11) or an accessory artery, arising from the SMA. An aberrant left hepatic artery exists in up to 25% of people. The left hepatic artery may be replaced to the SMA or may arise from the left gastric artery. Hepatic arteriography is most commonly performed today for trauma and neoplastic disease, usually as a precursor to endovascular intervention.
Trauma. In the setting of blunt or penetrating trauma, hepatic arteriography is used to evaluate for vascular injury in patients with abnormal CT or US. Typically, embolization is indicated in hemodynamically stable patients with hepatic arterial injury and evidence of bleeding, or in patients that present with a delayed complication from conservative management of a liver laceration. Iatrogenic injuries from biopsies or percutaneous cholangiography with or without drainage occur and can be resolved with endovascular treatment. Indications for embolization in the acute setting include continued hemodynamic instability, arterioportal fistula, and hemobilia (Fig. 24.12). In the delayed setting, pseudoaneurysm discovered on CT or US carries a 44% risk of rupture and may require emergent management.
Neoplasms are diagnosed by CT, MR, and US. Angiography is performed to determine resectability, to provide an arterial roadmap, or to deliver transcatheter therapy such as embolization.
Capillary hemangiomas demonstrate uniform dense stain in the late arterial phase, which persists beyond the venous phase. They usually have well-defined (but irregular) borders with a feeding artery, which is near normal in size. Cavernous hemangiomas have the classic appearance of contrast puddles near the periphery in well-marginated vascular spaces while the stain persists beyond the venous phase. The lesions may be up to 15 cm in size. The feeding artery is usually normal in size. Hemangioendotheliomas present in infancy either with mass effect or hepatomegaly. Most (90%) are associated with extrahepatic hemangiomas (cutaneous lesions). The lesion usually involutes within 1 to 2 years. Treatment may be required if the lesions cause symptoms. Angiography shows dilated irregular vascular lakes, staining beyond the venous phase, and dilated feeding vessels. Arteriovenous shunting and early opacification of hepatic veins is also described.
Angiography of hepatocellular carcinoma (HCC) demonstrates a hypervascular mass with large distorted feeding arteries. Neovascularity and intratumoral puddling of contrast and portal vein invasion with arterioportal shunting may be demonstrated. Up to 25% of tumors are hypovascular. The combination of portal venous invasion and arterioportal shunting is virtually pathognomonic for HCC. Angiography of cholangiocarcinoma demonstrates a hypovascular or avascular tumor without neovascularity. The most common malignant liver lesions are metastases. As shown on physiological studies, the degree of vascularity and staining on angiography has little relation to tumor blood flow. Even with hypovascular metastases, the blood flow is increased relative to normal liver parenchyma. Angiography may show displacement of adjacent vessels and compressed or occluded portal veins. Arterial encasement or shunting is rare. Embolization of metastases has mixed results. Hypervascular metastases include neuroendocrine tumor, renal cell carcinoma, thyroid carcinoma, and choriocarcinoma. Hypovascular metastases include lung, esophagus, and pancreas carcinoma. Mixed vascularity is seen in breast carcinoma, ocular carcinoma, cholangiocarcinoma, and sarcoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree