Pancreas and Spleen
William E. Brant
Pancreas
Imaging Techniques
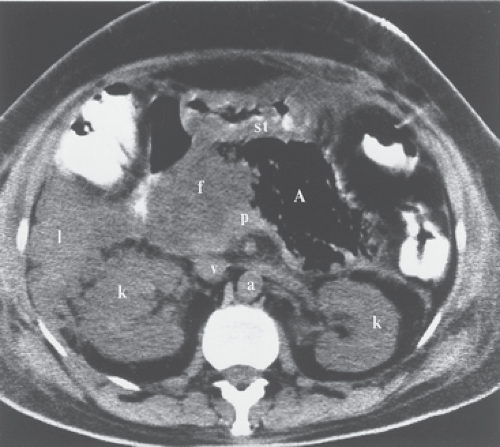
CT, US, and MR provide high-quality images of the pancreatic parenchyma and are used as the primary imaging modalities for the pancreas (Fig. 27.1). MDCT optimizes contrast enhancement for detection of small tumors and provides the capability of CT angiography to detect vascular involvement by pancreatic tumor. Improved MR techniques and the use of gadolinium enhancement have increased its capability to detect and characterize pancreatic lesions (1). MR cholangiopancreatography (MRCP) offers an excellent noninvasive method of imaging the pancreatic duct as well as the biliary system. Secretin administration during MRCP (secretin test) increases pancreatic secretions and improves visualization of the pancreatic duct (2). Endoscopic retrograde cholangiopancreatography (ERCP) provides excellent visualization of the lumen of the pancreatic duct (Fig. 27.2), which is usually affected by any mass lesion of the pancreas. This procedure is performed by endoscopic cannulation of the bile and the pancreatic ducts, followed by injection of a contrast agent and radiography. Arteriography is now routinely performed using CT and MR angiographic techniques (CTA, MRA). US- and CT-guided biopsy and drainage procedures play a major role in the diagnosis and treatment of pancreatic diseases. Endoscopic US is an important adjunct to characterize pancreatic tumors by imaging and endoscopic US-guided fine needle aspiration (3).
Anatomy
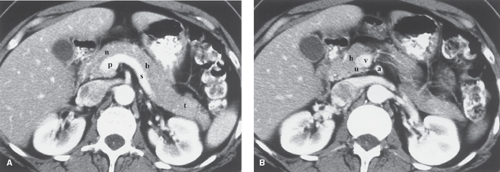
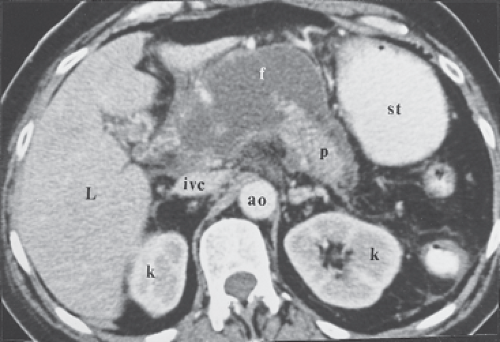
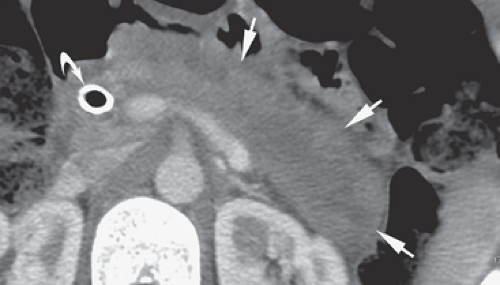
The pancreas is a tongue-shaped organ, approximately 12 to 15 cm in length, that lies within the anterior pararenal compartment of the retroperitoneum (4) (Fig. 27.1). The pancreas is posterior to the left lobe of the liver, the stomach, and the lesser sac. It is anterior to the spine, the inferior vena cava, and the aorta. Pancreatic tissue is best recognized by identification of the vessels around it. The neck, body, and tail of the pancreas lie ventral to the splenic vein, with the tail extending into the hilum of the spleen. The splenic vein and pancreas are anterior to the superior mesenteric artery. The head of the pancreas wraps around the junction of the superior mesenteric vein and the splenic vein, with the uncinate process of the pancreatic head extending under the superior mesenteric vein just anterior to the inferior vena cava. The splenic artery courses through the pancreatic bed in a tortuous course. Atherosclerotic splenic artery calcifications are easily mistaken for pancreatic calcifications. The lumen of the splenic artery may be mistaken for pancreatic cysts or a dilated pancreatic duct on a CT without contrast or US.
Maximum dimensions for pancreatic size are 3.0 cm diameter for the head, 2.5 cm diameter for the body, and 2.0 cm diameter for the tail. The gland is somewhat larger in young patients and progressively decreases in size with age. Because the gland is not encapsulated, fatty infiltration between the lobules in older patients gives the pancreas a delicate, feathery appearance on the CT. The pancreatic duct is visualized with thin-slice CT and with US. It normally measures 3 to 4 mm in diameter in the head and tapers smoothly toward the tail. Images from ERCP show the normal duct to be a bit larger owing to magnification effect and distension resulting from contrast injection (Fig. 27.2). The duodenum cradles the pancreatic head in the C-loop. Many pancreatic abnormalities show secondary effects on the duodenum and occasionally on the stomach and the colon.
On MR, the pancreas is well seen on fat-suppressed T1WI. High protein content in the exocrine pancreas results in high signal of the pancreatic parenchyma, which is difficult to differentiate from fat on non-fat-suppressed T1WI. Tumors are typically of lower signal than pancreatic parenchyma on T1WI. On T2WI, pancreatic tissue is variable in signal intensity from as low as the liver is to as high as fat. Cystic lesions are bright and easily seen on T2WI. Gadolinium will enhance the parenchyma, whereas adenocarcinoma enhances poorly and remains a low signal on postcontrast T1WI.
Pancreatitis
Acute pancreatitis is generally diagnosed clinically. The role of imaging is to clarify the diagnosis when the clinical picture is confusing, to assess its severity, to determine prognosis, and to detect complications. Inflammation of the pancreatic tissue leads to disruption of small pancreatic ducts, resulting in leakage of pancreatic secretions. Because the pancreas lacks a capsule, the pancreatic juices have ready access to the surrounding
tissues. Pancreatic enzymes digest fascial layers, spreading the inflammatory process to multiple anatomic compartments. Causes of acute pancreatitis are listed in Table 27.1 (5). Imaging studies of acute pancreatitis may be normal in mild cases. Contrast-enhanced MDCT provides the most comprehensive initial assessment; however, US is useful for follow-up of specific abnormalities such as fluid collections. Abnormalities that may be seen in the pancreas include (6) (1) focal or diffuse parenchymal enlargement, (2) changes in density due to edema, and (3) indistinctness of the margins of the gland due to inflammation. Abnormalities in the peripancreatic tissues include stranding densities in the fat with indistinctness of the fat planes and thickening of affected fascial planes. Complications demonstrated by imaging are listed in Table 27.2 (Figs. 27.3 to 27.5) (7). US-directed or CT-directed aspiration biopsy may be needed to confirm the presence of pancreatic abscess. Image-directed catheter placement is an alternative to surgical drainage of pancreatic fluid collections. Contrast-enhanced MR is equivalent to CT in the assessment of pancreatitis.
tissues. Pancreatic enzymes digest fascial layers, spreading the inflammatory process to multiple anatomic compartments. Causes of acute pancreatitis are listed in Table 27.1 (5). Imaging studies of acute pancreatitis may be normal in mild cases. Contrast-enhanced MDCT provides the most comprehensive initial assessment; however, US is useful for follow-up of specific abnormalities such as fluid collections. Abnormalities that may be seen in the pancreas include (6) (1) focal or diffuse parenchymal enlargement, (2) changes in density due to edema, and (3) indistinctness of the margins of the gland due to inflammation. Abnormalities in the peripancreatic tissues include stranding densities in the fat with indistinctness of the fat planes and thickening of affected fascial planes. Complications demonstrated by imaging are listed in Table 27.2 (Figs. 27.3 to 27.5) (7). US-directed or CT-directed aspiration biopsy may be needed to confirm the presence of pancreatic abscess. Image-directed catheter placement is an alternative to surgical drainage of pancreatic fluid collections. Contrast-enhanced MR is equivalent to CT in the assessment of pancreatitis.
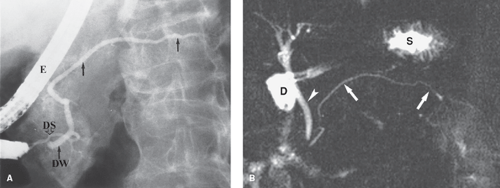
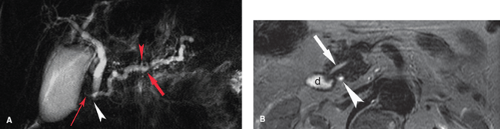
Pancreas divisum is a common congenital variant of pancreatic anatomy that serves as a predisposition to pancreatitis (Fig. 27.6) (2). The ventral and dorsal ductal systems of the pancreas fail to fuse. As a result, the major portion of the pancreatic secretions from the body and tail drain through the dorsal pancreatic duct (Santorini) into the minor papilla,
whereas the minor portion of pancreatic secretions from the head and uncinate process (ventral duct of Wirsung) drain into the duodenum through the major papilla in association with the common bile duct. Relative obstruction at the minor papilla results in pancreatitis in 5% to 15% of patients with pancreas divisum. The anomaly is found in 6% of the general population and in 10% to 20% of patients with a history of
acute recurrent pancreatitis. MRCP and ERCP are most reliably used to make the diagnosis.
whereas the minor portion of pancreatic secretions from the head and uncinate process (ventral duct of Wirsung) drain into the duodenum through the major papilla in association with the common bile duct. Relative obstruction at the minor papilla results in pancreatitis in 5% to 15% of patients with pancreas divisum. The anomaly is found in 6% of the general population and in 10% to 20% of patients with a history of
acute recurrent pancreatitis. MRCP and ERCP are most reliably used to make the diagnosis.
Table 27.1 Causes of Acute Pancreatitis | |
|---|---|
|
Table 27.2 Complications of Acute Pancreatitis | |
|---|---|
|
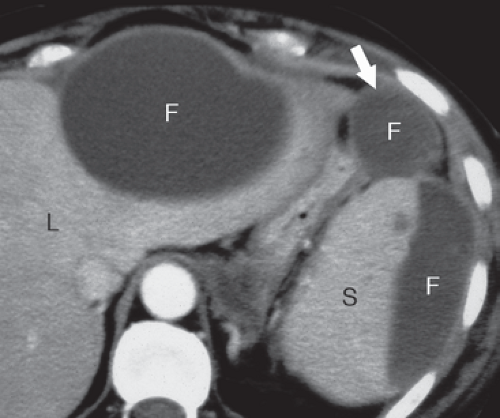
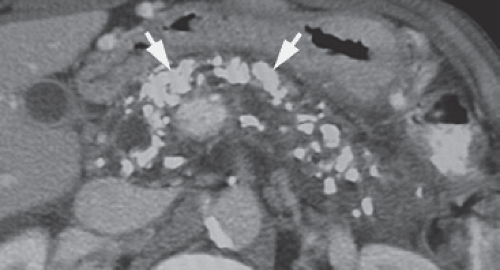
Chronic pancreatitis is caused by recurrent and prolonged bouts of acute pancreatitis that cause parenchymal atrophy and progressive fibrosis. Both the exocrine and the endocrine functions of the pancreas may be impaired. The most common causes are alcohol abuse (70%) and biliary stone disease (20%). Many of the remaining patients may have autoimmune pancreatitis that responds to steroid therapy. The clinical diagnosis is often vague; therefore, imaging is used both to confirm the diagnosis and to detect the complications. The morphologic changes of chronic pancreatitis include (8) (1) dilation of the pancreatic duct (70% to 90% of cases), usually in a beaded pattern of alternating areas of dilation and constriction (Fig. 27.7); (2) decrease in visible pancreatic tissue because of atrophy; (3) calcifications (40% to 50% of cases) in the pancreatic parenchyma that vary from finely stippled to coarse, usually associated with alcoholic pancreatitis (Fig. 27.8); (4) fluid collections that are both intrapancreatic and extrapancreatic; (5) focal mass-like enlargement of the pancreas owing to benign inflammation and fibrosis; (6) stricture of the biliary duct because of fibrosis or mass in the pancreatic head resulting in proximal bile duct dilatation; and (7) fascial thickening and chronic inflammatory changes in the surrounding tissues. Differentiation between an inflammatory mass resulting from chronic pancreatitis and that of pancreatic carcinoma often requires image-directed biopsy. MR reveals the fibrosis and parenchymal atrophy as a loss of the bright signal of pancreas parenchyma normally seen on T1-weighted fat-suppressed images. Parenchymal enhancement on MR is heterogeneous early and increases on delayed images. MRCP and ERCP demonstrate the characteristic changes in the pancreatic duct. Calcifications are demonstrated by CT, US, and plain radiographs but are easily overlooked on MR.
Autoimmune pancreatitis (lymphoplasmacytic sclerosing pancreatitis) is a unique form of chronic pancreatitis caused by autoimmune system disease that involves the pancreas, kidneys, bile ducts, and retroperitoneum (9). Periductal infiltration by lymphocytes and plasma cells results in mass-like enlargement of the pancreas closely simulating adenocarcinoma. Differentiation is important because autoimmune pancreatitis is effectively treated with oral steroids. Findings that favor
a diagnosis of autoimmune pancreatitis include (Fig. 27.9) (1) diffuse or focal swelling of the pancreas with characteristic tight halo of edema; (2) extensive peripancreatic stranding and edema are absent; (3) diffuse or segmental narrowing of the pancreatic duct or the common bile duct; (4) absence of dilatation of the pancreatic duct and absence of parenchymal atrophy proximal to the pancreatic mass (these findings are typically present with adenocarcinoma); (5) pseudocysts and parenchymal calcifications are typically absent, (6) peripancreatic blood vessels are usually not involved; (7) the kidneys are involved in one-third of cases showing round, wedge-like, or diffuse peripheral patchy areas of decreased contrast enhancement; and (8) serum IgG4 is often elevated (10).
a diagnosis of autoimmune pancreatitis include (Fig. 27.9) (1) diffuse or focal swelling of the pancreas with characteristic tight halo of edema; (2) extensive peripancreatic stranding and edema are absent; (3) diffuse or segmental narrowing of the pancreatic duct or the common bile duct; (4) absence of dilatation of the pancreatic duct and absence of parenchymal atrophy proximal to the pancreatic mass (these findings are typically present with adenocarcinoma); (5) pseudocysts and parenchymal calcifications are typically absent, (6) peripancreatic blood vessels are usually not involved; (7) the kidneys are involved in one-third of cases showing round, wedge-like, or diffuse peripheral patchy areas of decreased contrast enhancement; and (8) serum IgG4 is often elevated (10).
Imaging findings normalize following steroid treatment.
Groove pancreatitis is an uncommon form of chronic pancreatitis that may also mimic adenocarcinoma (5). Fibrosis in the groove between the head of the pancreas, the descending duodenum, and the common bile duct produces an inflammatory mass that obstructs the common bile duct. Characteristic findings include (1) sheet-like mass in the pancreaticoduodenal groove, (2) atrophy and fibrotic changes in the pancreatic head, (3) small cysts along the wall of the duodenum, (4) duodenal wall thickening and luminal narrowing, (5) tapering stenosis of the common bile and pancreatic ducts, and (6) widening of the space between the distal ducts and the wall of the duodenum (rarely seen with adenocarcinoma) (11).
Solid Lesions of the Pancreas
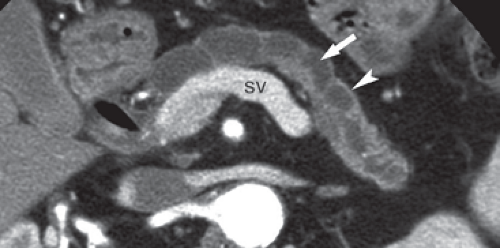
Pancreatic adenocarcinoma (ductal carcinoma) is a highly lethal tumor that is usually unresectable at presentation (12). The average survival time of a patient with this disease is only 5 to 8 months. It accounts for 3% of all cancers and is second only to colorectal cancer as the most common digestive tract malignancy. Radiographic assessment of resectability is critical because surgical resection offers the only hope of cure; yet, the surgery itself carries a high morbidity. Scanning by CT should include rapid bolus contrast injection, thin slices, and CT angiography to provide accurate tumor staging (13). Adenocarcinoma appears as a hypodense mass distorting the contour of the gland. Associated findings include obstruction of the common bile duct and the pancreatic duct and atrophy of pancreatic tissue beyond the tumor. Metastases commonly go to regional nodes, liver, and the peritoneal cavity (14). Signs of resectability (Fig. 27.10A) include (1) isolated pancreatic mass with or without dilation of the bile or pancreatic ducts, (2) no extrapancreatic disease, and (3) no encasement of celiac axis or superior mesenteric artery. Signs of potential respectability include (1) absence of involvement of the celiac axis or the superior mesenteric artery, (2) regional nodes may be involved, and (3) limited peripancreatic extension of tumor may be present. Signs of unresectability include (1) encasement of the celiac axis or the superior mesenteric artery (Fig. 27.10B), (2) occlusion of the superior mesenteric or portal
vein without a technical option for reconstruction, and (3) liver, peritoneal, lung, or any other distant metastases (13). Evidence of arterial encasement that indicate unresectability include (1) tumor abutting greater than 180° of the circumference of the artery, (2) tumor abutment focally narrowing the artery, and (3) occlusion of the artery by tumor (13,15,16). Only 5% to 30% of patients have tumors that are potentially resectable using these criteria. As noted previously, consideration should be given to alternative diagnoses of autoimmune or groove pancreatitis. Image-guided biopsy can confirm the diagnosis in patients whose tumors are deemed to be unresectable. Tumor recurrence following the Whipple procedure is best detected with MDCT. MR shows low-signal infiltrative tumor surrounded by high-signal-enhanced parenchyma on postcontrast T1WI. MRCP defines ductal anatomy with dilatation proximal to the stricturing tumor. MRA and MRV are excellent in identifying vascular involvement by tumor.
vein without a technical option for reconstruction, and (3) liver, peritoneal, lung, or any other distant metastases (13). Evidence of arterial encasement that indicate unresectability include (1) tumor abutting greater than 180° of the circumference of the artery, (2) tumor abutment focally narrowing the artery, and (3) occlusion of the artery by tumor (13,15,16). Only 5% to 30% of patients have tumors that are potentially resectable using these criteria. As noted previously, consideration should be given to alternative diagnoses of autoimmune or groove pancreatitis. Image-guided biopsy can confirm the diagnosis in patients whose tumors are deemed to be unresectable. Tumor recurrence following the Whipple procedure is best detected with MDCT. MR shows low-signal infiltrative tumor surrounded by high-signal-enhanced parenchyma on postcontrast T1WI. MRCP defines ductal anatomy with dilatation proximal to the stricturing tumor. MRA and MRV are excellent in identifying vascular involvement by tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree