Mesenteric Small Bowel
William E. Brant
Imaging Methods
Disease of the mesenteric small intestine is relatively rare (1,2). A detailed radiographic study of the small bowel is justified only when the clinical suspicion of small bowel disease is high. Small bowel disease is usually manifest by four major symptoms: colic, diarrhea, malabsorption, and bleeding. Colic is defined as recurrent and spasmodic abdominal pain with periods of relief every 2 to 3 minutes. Diarrhea caused by small bowel disease is less urgent than that caused by colon disease. Malabsorption is manifest by steatorrhea, foul-smelling stools, and weight loss. Bleeding from small bowel disease is usually occult and manifest by anemia. The majority of the mesenteric small intestine is out of traditional reach of the endoscopist, giving diagnostic radiology a primary role in its evaluation. The development of capsule endoscopy provides a limited but safe and well-accepted method for small bowel endoscopy. Traditional fluoroscopic methods of small bowel evaluation are being supplemented and replaced by CT and MR enteroclysis and enterography. Fluoroscopic methods are limited to the evaluation of the lumen of the small bowel, whereas the cross-sectional methods of CT and MR provide added information about the wall of the small bowel, its mesentery, and adjacent structures and tissues.
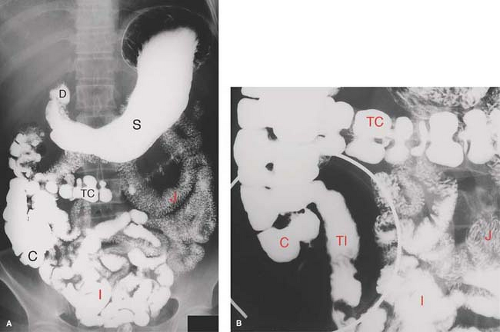
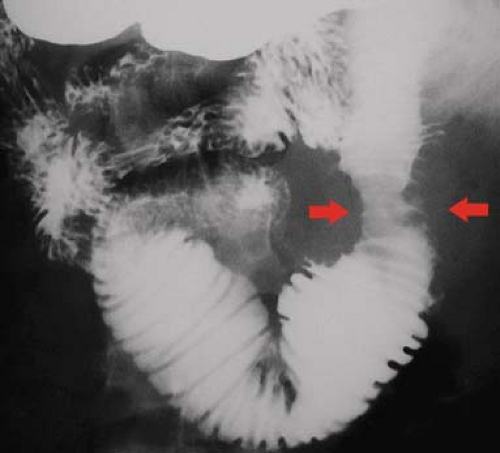
Small bowel follow-through (SBFT) is the traditional method (Fig. 30.1) for radiographic examination of the small bowel tacked onto a standard upper GI (UGI) series. The patient is asked to continue drinking barium while a series of supine abdominal films are obtained until the terminal ileum and cecum are filled with barium. Fluoroscopic examination of the small bowel is then performed. This study is notoriously insensitive. It is limited by overlap of bowel loops, poor distension, flocculation of barium, intermittent barium filling, and unpredictable transit time. Visualization of the distal ileum may be improved with a double-contrast technique by insufflating the colon with air (SBFT with peroral pneumocolon).
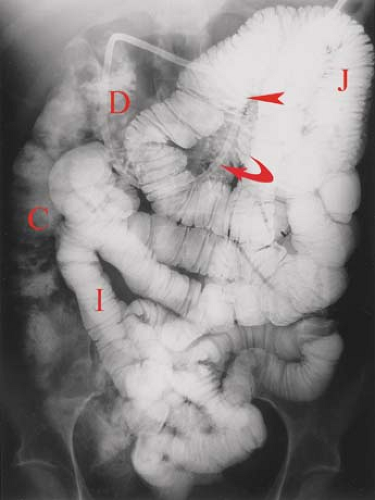
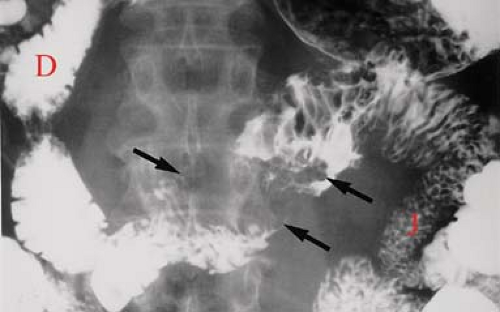
Enteroclysis, or the small bowel enema, is a more sensitive fluoroscopic method for detailed small bowel examination (Fig. 30.2). This study provides more uniform distension of the bowel, even distribution of barium, superior anatomic detail, and shorter overall examination time (1). The study is performed by passing a specially designed 12 to 14 French enteroclysis catheter through the mouth or nose and into the distal duodenum or proximal jejunum. A guidewire is used for directional control of the catheter during manipulation under fluoroscopy. The study may be performed single contrast using approximately 600 mL of barium or double contrast using 200 mL of barium followed by 1000 mL of methylcellulose to advance the barium and distend the bowel. The small bowel lumen and mucosal surface are best demonstrated by barium studies.
CT enteroclysis improves upon barium enteroclysis by demonstrating the extraluminal component of bowel disease, the mesentery, adjacent solid organs, the peritoneal cavity, and the retroperitoneum (3). Patients prepare with a low residue diet on the day before the examination followed by an overnight fast. Similar to fluoroscopic enteroclysis, an 8 to 13 French nasojejunal catheter is advanced beyond the ligament of Treitz under fluoroscopic guidance. A choice is made between using high-attenuation enteric contrast agents without IV contrast agents and using low-attenuation enteric contrast agents with IV contrast enhancement. High-attenuation contrast agents include 4% to 15% water-soluble iodinated contrast agents and dilute barium solution. Low-attenuation enteric agents include water and methylcellulose. Two liters of enteric agent is infused at 100 to 150 cc/min under fluoroscopic observation. Glucagon or other antispasmodic agent is administered intravenously. The patient is moved to the CT table and an additional 500 to 1000 cc of enteric contrast is infused at the same rate during CT scanning. Thin-slice MDCT allows for high-resolution reconstructions in axial, coronal, and sagittal planes.
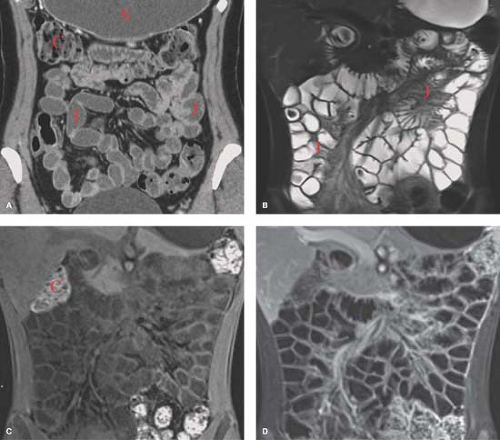
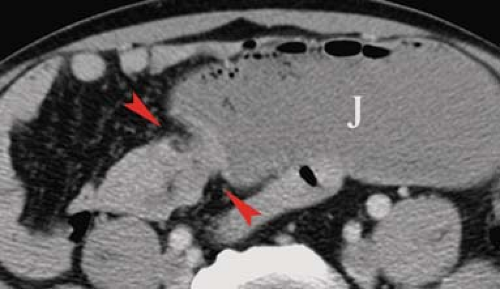
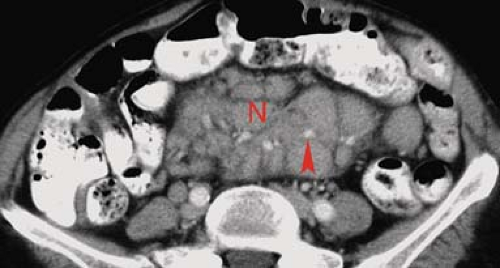
CT enterography is performed in a manner similar to CT enteroclysis except the 1.5 to 2.0 L of enteric contrast is given orally instead of by enteric tube injection (Fig. 30.3). Either high-attenuation or low-attenuation enteric contrast agents may be used. Low-attenuation enteric agents allow for the use of IV contrast to assess bowel wall and lesion enhancement. CT enterography tends to have less reliable and less complete distension of the small bowel but is easier to perform and has higher patient acceptance.
MR enteroclysis and MR enterography are performed in a similar manner to CT enteroclysis and CT enterography (Fig. 30.3) (4). While more expensive and somewhat less available MR small bowel studies offer the significant advantage of lack of use of ionizing radiation. This is particularly important in the study of patients with Crohn disease who are young and undergo many imaging examinations. Tissue contrast is also superior with MR. MR enterography is most commonly used with MR enteroclysis reserved for patients with low-grade small bowel obstruction or who are unable to ingest large volumes of enteric agents orally. A wide variety of enteric agents are available, but the most popular are biphasic agents
that are of low-signal intensity on T1WI and of high-signal intensity on T2WI. Biphasic agents include water, methylcellulose, low-density barium, and polyethylene glycol. Patients are asked to ingest 1200 to 2000 cc of enteric agent in the hour before MR scanning. Spasmolytic agents reduce peristalsis and motion artifacts. Breath hold fast gradient echo sequences are obtained in axial, sagittal, and coronal planes. IV contrast may be utilized to assess for inflammatory hyperenhancement and tumor vascularity. Preliminary studies using state-of-the-art techniques indicate equivalent sensitivities for CT enterography and MR enterography (2,5). Diagnostic findings of small bowel disease on MR and CT are listed in Table 30.1.
that are of low-signal intensity on T1WI and of high-signal intensity on T2WI. Biphasic agents include water, methylcellulose, low-density barium, and polyethylene glycol. Patients are asked to ingest 1200 to 2000 cc of enteric agent in the hour before MR scanning. Spasmolytic agents reduce peristalsis and motion artifacts. Breath hold fast gradient echo sequences are obtained in axial, sagittal, and coronal planes. IV contrast may be utilized to assess for inflammatory hyperenhancement and tumor vascularity. Preliminary studies using state-of-the-art techniques indicate equivalent sensitivities for CT enterography and MR enterography (2,5). Diagnostic findings of small bowel disease on MR and CT are listed in Table 30.1.
Capsule endoscopy involves the use of a swallowable video capsule 26 mm long by 11 mm diameter and weighing 4 g. The capsule contains a video camera, four light-emitting diodes as light source, a radio transmitter, and batteries (1). Patients fast for 10 hours prior to ingesting the capsule. A sensor array is placed on the patient’s abdomen and attached to a portable battery-powered recorder that can be worn around the waist. The capsule is swallowed, and color video images are recorded at the rate of two per second up to approximately 50,000 images over an 8-hour battery life span. The patient resumes normal activities including eating while the capsule transits the intestinal tract. The capsule is excreted naturally and discarded. Capsules cost approximately $1500. Images are reviewed on a workstation. Capsule endoscopy is able to visualize the entire small bowel mucosa and may detect mucosal lesions, ulcers, and tumors missed by imaging examinations. Significant limitations include limited ability to localize, biopsy, or treat lesions and limited use in patients with small bowel obstruction or strictures.
Table 30.1 Diagnostic Findings on Ct and Mr of the Gi Tract | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Anatomy
The mesenteric small intestine is a tube approximately 7-m long that lies totally within the greater peritoneal cavity. The jejunum is arbitrarily defined as the proximal two-fifths of the mesenteric intestine, whereas the ileum is the distal three-fifths. The jejunum and ileum are suspended from the posterior abdominal wall by the small bowel mesentery. The small bowel mesentery is composed of connective tissue, blood vessels, and lymphatic vessels and is covered by peritoneum, which reflects from the posterior parietal peritoneum. The root of the small bowel mesentery extends obliquely from the ligament of Treitz, just left of the L-2 vertebra, to the cecum, near the right sacroiliac joint (6). On CT, the mesentery is defined by its normal vascular structures outlined by fat between loops of bowel. Normal mesenteric lymph nodes may be seen as soft-tissue density nodules 5 mm or less in size. The concave border of the small bowel loops is the mesenteric border where the mesentery attaches. The convex border, facing away from the mesentery, is called the antimesenteric border.
Identification of the border involved by disease can be of diagnostic value.
Identification of the border involved by disease can be of diagnostic value.
Table 30.2 Normal Small Bowel Measurements | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
On imaging studies (Figs 30.1 to 30.3), the jejunum has a feathery mucosal pattern, more prominent valvulae conniventes, a wider lumen, and a thicker wall. The ileum has a less featured mucosal pattern, thinner, less frequent folds, narrower lumen, and a thinner wall. The transition between jejunum and ileum is gradual, and all loops are freely mobile. The ileum has larger and more numerous lymphoid follicles in the submucosa. Villi are finger-like projections that extend from the entire mucosal surface of the small bowel. They are composed of loose connective tissue of the lamina propria. Tiny capillaries and lymphatic vessels (lacteals) extend to the submucosal vessels. The combination of valvulae conniventes and villi greatly expands the absorptive surface area of the small intestine. The caliber of the normal small bowel lumen is less than 3 cm in the jejunum tapering to less than 2 cm in ileum (Table 30.2) (7). Normal jejunal folds measure 2 to 3 mm thick, whereas normal ileum folds measure 1 to 2 mm thick. Enteroclysis typically distends the normal jejunum to 4 cm and the normal ileum to 3 cm, with the folds appearing 1 mm thinner in each portion of the mesenteric small bowel. Normal lymph nodes seen in the mesentery are less than 4 mm in diameter.
Small Bowel Filling Defects/Mass Lesions
Neoplasms of the small intestine are rare, accounting for only 2% to 3% of GI tumors (8). Benign neoplasms are about equal to malignant neoplasms in overall frequency. However, when the patient presents with symptoms, malignancy is three times more common. Presenting afflictions include obstruction, pain, weight loss, bleeding, and palpable mass. CT and MR enterography findings that suggest malignant small bowel lesions include (1) solitary lesions, (2) nonpedunculated lesions, (3) long segment lesions, (4) presence of mesenteric fat infiltration, and (5) presence of enlarged mesenteric lymph nodes (>1 cm short axis diameter) (9).
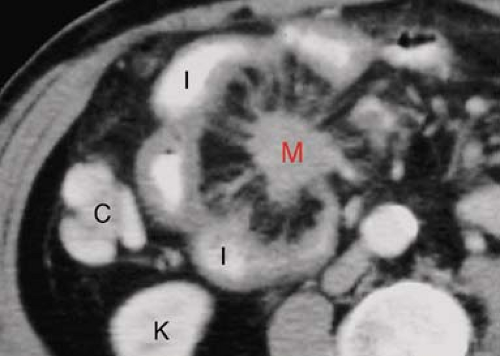
Carcinoid tumors are the most common neoplasm of the small intestine, accounting for about one-third of all small bowel tumors (10). They are considered a low-grade malignancy that may recur locally or metastasize to the lymph nodes, liver, or lung. They arise from the endocrine cells (enterochromaffin or Kulchitsky cells) deep in the mucosa. These cells produce vasoactive substances including serotonin and bradykinins. About 20% of all carcinoid tumors arise in the small bowel, most commonly in the ileum where 30% are multiple. Only 7%, those with liver metastases, present with carcinoid syndrome (cutaneous flushing, abdominal cramps, and diarrhea) because the liver inactivates the vasoactive substances. The tumors grow slowly but cause a marked fibrotic response of the bowel wall and mesentery because the serotonin produced by the tumor induces an intense local desmoplastic reaction. Complications include stricture, obstruction, and bowel infarction induced by fibrosis of the mesenteric vessels. The tumors may be pedunculated and cause intussusception. Radiographic signs of fibrosis and metastases resemble the findings of Crohn disease and overshadow the demonstration of the primary tumor. Barium studies show (1) luminal narrowing, (2) thickened and spiculated folds, (3) separation of bowel loops by mesenteric mass or (4) bowel loops drawn together by fibrosis, and (5) primary lesion appearing as small (<1.5 cm) mural nodule or intraluminal polyp. CT and MR findings that are highly indicative of carcinoid tumor are (Fig. 30.4) (1) sunburst pattern of radiating soft-tissue density in the mesenteric fat due to mesenteric fibrosis, (2) bowel wall thickening, (3) primary lesion appearing as a small, lobulated soft-tissue mass, occasionally with central calcification, usually in the distal ileum, (4) marked contrast enhancement of the primary tumor mass; and (5) enlarged mesenteric nodes and liver masses due to metastatic disease (8).
Adenocarcinoma of the small bowel is about half as common as carcinoid tumor. It is most frequent in the duodenum (50%) and proximal jejunum and is uncommon in the distal ileum, where carcinoid is most common. Most patients are symptomatic at presentation, and 30% have a palpable mass. Patients with adult celiac disease, Crohn disease, and Peutz–Jeghers syndrome are at increased risk for small bowel carcinoma. Complications include bleeding, obstruction, and intussusception. Prognosis is poor, with a 5-year survival of 20%. Metastatic spread is by intraperitoneal seeding, lymphatic channels to regional nodes, and portal veins to the liver. Morphologically, the tumor may be infiltrating producing strictures, polypoid producing filling defects, or ulcerating. Barium studies typically show a characteristic “apple core” stricture of the small bowel (Fig. 30.5). CT and MR (Fig. 30.6) demonstrate (1) a solitary mass in the duodenum or jejunum (up to 8 cm diameter), (2) an ulcerated lesion, or (3) an abrupt irregular circumferential narrowing of the bowel lumen with abrupt edges to the wall thickening. Differential diagnosis
of annular constricting lesions of the small bowel is listed in Table 30.3.
of annular constricting lesions of the small bowel is listed in Table 30.3.
Table 30.3 Annular Constricting Lesions | |||||
|---|---|---|---|---|---|
|
Lymphoma is responsible for about 20% of all small bowel malignant tumors. The GI tract is the most common site for extranodal origin of lymphoma, and the small bowel is most commonly involved. Most cases are non–Hodgkin lymphoma of B-cell type. Non-Hodgkin lymphoma clinically involves the GI tract in 30% of cases overall. Lymphoma is most frequent in the distal ileum where the concentration of lymphoid tissue is the greatest. Morphologic patterns of involvement include diffuse infiltration, exophytic mass, polypoid mass, and multiple nodules. Multiple sites of involvement are seen in 10% to 25% of cases. Aneurysmal dilation of the lumen is a feature of lymphoma due to the replacement of the muscularis and destruction of the autonomic plexus by tumor without inducing fibrosis. As a result, obstruction is uncommon. Barium studies most commonly reveal (1) wall thickening with irregular, distorted folds due to submucosal infiltration of cells (Fig. 30.7); (2) fold thickening may be smooth and regular in early stages due to lymphatic blockage in the mesentery; (3) folds become effaced in later stages with greater cell infiltration into the bowel wall; (4) narrowed, widened, or normal lumen; (5) cavitary lesions containing fluid and debris; (6) polypoid masses that may cause intussusception; and (7) rare multiple filling defects that are larger than 4 mm, variable in size, and nonuniform in distribution. Shallow ulceration is common. CT demonstrates (1) circumferential wall thickening involving a long segment of small bowel, (2) effacement of folds, (3) mucosal nodularity, and (4) eccentric wall thickening (Fig. 30.8). Exophytic lymphoma is generally of uniform soft-tissue density and enhances little, if any, with IV contrast administration. This is a differentiating finding in comparison with GI stromal tumors (GISTs) and adenocarcinoma, which usually enhance prominently. CT and MR readily demonstrates associated findings of lymphoma including mesenteric and retroperitoneal adenopathy and hepatosplenomegaly (11). The mesentery may show a large confluent mass encasing multiple bowel loops or enlarged individual nodes (Fig. 30.9). The
“sandwich sign” refers to the sparing of rind of fat surrounding mesenteric vessels that are encased by lymphomatous nodes.
“sandwich sign” refers to the sparing of rind of fat surrounding mesenteric vessels that are encased by lymphomatous nodes.
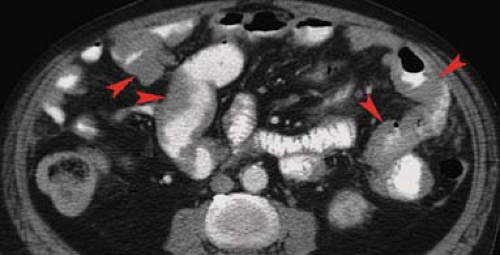 Figure 30.8. Non–Hodgkin Lymphoma—CT. CT image shows eccentric wall thickening (arrowheads) of multiple loops of small bowel. |
Burkitt lymphoma in North America usually presents with intestinal involvement, especially of the ileocecal area in children and young adults. The malignancy is aggressive, with rapid doubling time and poor prognosis. Imaging studies show bulky tumors.
AIDS-related lymphoma is an aggressive high-grade non–Hodgkin lymphoma with poor prognosis. Extranodal involvement, including small bowel lymphoma, is common. Adenopathy may be caused by lymphoma, Kaposi sarcoma, or Mycobacterium avium-intracellulare infection. The radiographic findings are identical to those seen in immunocompetent patients.
Nodular lymphoid hyperplasia may involve the entire small bowel. The condition is differentiated from lymphoma by the uniform small size of the nodules (2 to 4 mm) and even distribution through the area of involvement. Lymphoid hyperplasia confined to the terminal ileum and proximal colon is usually considered incidental and may be related to recent viral infection. Diffuse lymphoid hyperplasia is associated with hypogammaglobulinemia, especially low IgA.
Metastases to the small bowel are common (12). The two most frequent routes are by peritoneal seeding, usually involving the mesenteric border, and by hematogenous spread, which usually implants on the antimesenteric border. Intraperitoneal implantation on the small bowel serosa is most commonly due to ovarian carcinoma in women and colon, gastric, and pancreatic carcinoma in men. The mesenteric border of the small bowel is favored by the flow of fluid along the small bowel mesentery from the left upper to the right lower abdomen. Implantation is most common along the terminal ileum, cecum, and ascending colon. Peritoneal implants on the parietal peritoneum, and omentum (omental cake), as well as in the pouch of Douglas, are demonstrated by CT. Barium studies demonstrate nodules and tethering of folds due to mesenteric fibrosis. Hematogenous metastases are deposited along the antimesenteric border where the submucosal blood vessels arborize. Common primary malignancies are melanoma, lung, breast, and colon carcinoma, and embryonal cell carcinoma of the testes. Imaging studies demonstrate mural nodules of uniform or varying size anywhere in the small bowel. They may appear as target lesions, or ulcerate or cavitate. Direct extension to involve the small bowel is seen with malignancies of the pancreas and colon (Fig. 30.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree