■
Introduction
The aorta is the largest artery in the body, arising from the left ventricular outflow tract and branching throughout the body. The spectrum of thoracic aortic disease is wide, with causes that may be chronic or acute, congenital or acquired, and symptomatic or asymptomatic.
The clinical presentation of acquired thoracic aortic disease ranges from asymptomatic (as in the case of an ascending thoracic aortic aneurysm) to severe acute chest pain (in the case of aortic dissection). Technologic advances in multidetector computed tomography (MDCT), as well as increasing availability of advanced imaging, has increased clinical awareness of aortic diseases and has largely replaced direct catheter angiography as the method of aortic imaging.
Determining Who Needs Imaging
In the setting of acute chest pain, basic imaging is often performed, and cross-sectional advanced imaging may be pursued, depending on the degree of clinical suspicion for aortic disease. Imaging is also often performed in the setting of acute trauma (blunt or penetrating thoracic trauma); imaging modalities may be chosen based on the mechanism of trauma and/or degree of clinical suspicion for vascular injury.
Additionally, chronic aortic diseases may be followed with serial examinations. These imaging studies are often performed for surveillance of diseases (e.g., a thoracic aortic aneurysm), in which changes or increases in size may prompt more aggressive or surgical management.
■
Imaging Techniques
Radiography
Chest radiographs are often the first radiologic examination performed in the setting of acute or chronic thoracic aortic disease. The main utility of chest radiography resides in the frontal projection, and it is often used as a screening tool for the exclusion of acute aortic disease. However, radiography plays a limited role in the detailed workup of thoracic aortic diseases, mainly because of the limited anatomic detail and poor contrast resolution.
Advantages of Radiography
Chest radiography has the distinct advantage of being quick, readily available, and portable. Radiography plays a central role in the initial evaluation of an unstable patient or in a patient who cannot be easily positioned or moved. Frontal chest radiography may serve as a useful screening tool because a normal chest radiograph has a 98% negative predictive value in the setting of blunt trauma.
Although radiography uses ionizing radiation, the dose is relatively low. In cases when aortic disease has already been characterized, radiography may be a useful tool for surveillance by detecting change over time. Plain film radiography is useful for the detection of calcifications (particularly atherosclerotic calcifications), especially in the aorta and in valve leaflets or annuli. It is also helpful in the surveillance of support devices and localization of prosthetic devices (e.g., prosthetic valves, vascular endografts, pacemaker leads).
Disadvantages and Limitations of Radiography
Radiography has many limitations, mostly owing to the two-dimensional acquisition of images and low contrast resolution. This limits accurate and reproducible measurements of the mediastinum.
Although radiography may demonstrate the outward contours of the mediastinum, abnormalities of the intima are not apparent. As such, acute abnormalities such as dissection, penetrating aortic ulcer (PAU), and intramural hematoma (IMH) are usually not apparent radiographically.
Radiography also has the disadvantage of artifacts secondary to positioning, inspiratory effort, and projection. As an example, anterior-posterior imaging magnifies the size of the mediastinum more than a posterior-anterior image. Additionally, supine positioning or hypoinflation can artificially make the cardiomediastinal contours appear larger. Consequently, measurements and comparisons may be inaccurate owing to the lack of reproducible images.
Problem Solving With Radiography
Radiography of the chest is typically performed with the patient in an upright position, with posterior-anterior and lateral projections. This minimizes distortion of the mediastinal contours and artifacts related to positioning. However, in the emergent setting (e.g., spinal trauma, acute chest pain, hemodynamic instability), this may not be possible. In these cases, anterior-posterior projection radiography may be substituted.
Although relatively insensitive, the chest radiograph may display signs of acute aortic pathology. For example, mediastinal widening or obscuration of the normal aortic contours may suggest an aortic abnormality. And, although rare, inward displacement of atherosclerotic calcifications is virtually diagnostic of acute aortic dissection. Although these cases almost always proceed to cross-sectional imaging for further characterization, abnormal findings on the radiograph may be the first warning sign of serious aortic pathology.
Computed Tomography
CT was introduced in the early 1990s, providing a noninvasive alternative to catheter-directed angiography. Early iterations of CT used single detectors, requiring extended acquisition times and prohibitively long breath-holds. Additionally, the first iodinated contrast agents were hyperosmolar and associated with high rates of adverse reactions.
Since the advent of MDCT, acquisition times have significantly decreased, allowing for imaging to be obtained in a single breath-hold. Furthermore, thin slices and reconstruction software have allowed for isovolumetric multiplanar reconstruction at the radiologist’s workstation. Newer contrast agents are low osmolar and associated with lower rates of allergic reactions and renal injury.
Advantages of CT
CT is a relatively quick examination that allows for accurate and reproducible three-dimensional (3D) images of the aorta. With modern MDCT, multiplanar reconstructions can be made to evaluate and characterize the aorta accurately.
Actual image acquisition for a CT of the thoracic aorta can be performed in a matter of seconds, particularly with the advent of newer technology, such as dual-source scanners. However, overall, CT is more time consuming than radiography because it requires patient preparation (securing intravenous access), scanner setup, and time for image postprocessing. Nevertheless, the complete process of CT imaging from start to finish can be completed in a matter of minutes. This allows for noninvasive imaging of critically ill patients and can expedite management and treatment decision making. A secondary advantage to the fast acquisition of CT pertains to pediatric patients or patients in pain—short acquisition times are easier to tolerate and avoid motion artifacts from patient discomfort.
Disadvantages and Limitations of CT
An inherent disadvantage of CT imaging is the ionizing radiation associated with image acquisition. The radiation dose is of particular concern when imaging is performed in pediatric patients or when serial examinations are performed for disease surveillance.
Additionally, diagnostic imaging of the aortic lumen requires administration of IV contrast. However, IV contrast is contraindicated in the case of severe chronic renal insufficiency (glomerular filtration rate <30); in these cases, contrast-enhanced CT imaging cannot be performed. It should be mentioned, however, that patients with end-stage renal disease may receive iodinated contrast if hemodialysis is performed after contrast administration.
Furthermore, allergies to iodinated contrast are not uncommon, and the use of iodinated contrast may be contraindicated (e.g., in the case of anaphylaxis). Even in cases of mild allergy, premedication takes between 7 and 13 hours, which is usually not feasible in the assessment of traumatic injury or acute chest pain.
Although CT has a relatively short acquisition time, motion artifacts, including cardiac motion and breathing, may result in an artifact that degrades images. However, newer scanners with dual-source technology and more detectors may reduce artifacts and decrease scan times.
Problem Solving With Noncontrast CT
The noncontrast phase is an essential part of every examination of the aorta. Noncontrast evaluation allows for the characterization of intimal atherosclerotic calcifications, which may be obscured by contrast in an angiographic phase. Similarly, surgical material such as sutures or pledgets are intrinsically dense on CT; without a precontrast image, these can be mistaken for contrast leak or pseudoaneurysm.
Importantly, an acute IMH is most evident on noncontrast CT. This is seen as a crescentic hyperdensity tracking along the vessel wall. However, this may be obscured on the angiographic phase or may be mistaken for atheromatous plaque or mural thrombus instead of acute pathology.
The radiation dose may be reduced by a number of methods. Automated tube current modulation should always be used, when available. In the case of thin or pediatric patients, lower tube kVp may also be used. Newer scanners with dual-source technology can have faster image acquisition at lower radiation doses. Dual-source technology has also been used in virtual noncontrast imaging, eliminating the dose from an additional CT sequence. However, this technology is relatively new and is not universally available. Newer software using iterative reconstruction (as opposed to filtered backprojection) can reduce image noise, allowing for diagnostic images with lower radiation doses.
Problem Solving With Contrast-Enhanced CT
Contrast-enhanced CT angiography (CTA) is the mainstay for the emergent evaluation of acute aortic diseases. Contrast is used for the opacification of the vessel lumen, which allows for targeted evaluation of the intima.
Contrast is injected intravenously at a rate of 3 to 5 mL/s, usually followed by a saline chaser that allows contrast advancement through the circulatory system while decreasing contrast volume. Bolus-tracking strategy is generally used, with a region of interest set over the blood pool (typically the ascending aorta). When the density of the ascending aorta has reached a threshold, scanning begins.
In some cases, a delayed scan may be performed 2 minutes after injection of contrast. Delayed scans may assess for late contrast opacification of a false lumen (in the case of aortic dissection) or delayed opacification of an endoleak in the case of endograft repair. Additionally, delayed images may demonstrate contrast extravasation that is not apparent on angiographic phase.
Nearly all examinations are subject to postprocessing, including multiplanar (axial, sagittal, and coronal) reformats and maximum intensity projection images. The additional use of interactive reformatting software can allow for oblique reformats (e.g., candy cane view of the thoracic aorta). Three-dimensional surface rendering of the aorta can aid in the additional characterization of thoracic disease and treatment planning.
Magnetic Resonance Imaging
MRI is an imaging method that does not use ionizing radiation and provides an alternative to the use of CT in cross-sectional thoracic imaging. MRI uses a combination of a strong magnetic field and radiofrequency pulses to create cross-sectional images. In contrast to CT, this requires multiple sequences (image acquisitions). The most useful tool in aortic imaging by MRI is usually contrast-enhanced (using gadolinium-based contrast agents) magnetic resonance angiography (MRA). However, some MRA sequences may also be obtained using noncontrast protocols, which are useful in cases where gadolinium contrast is contraindicated.
Advantages of MRI
Magnetic resonance imaging has the distinct advantage of providing cross-sectional images without the use of ionizing radiation. This is ideal for many situations, particularly in the case of young patients or patients who need serial studies for surveillance. MR images provide multiplanar reformatted images, which may be adjusted to any plane that is tailored to any patient’s individual anatomy. In addition to anatomic information, MRI can provide functional imaging through the use of cinematic and phase contrast sequences.
Another advantage of MR over CT is the use of contrast agents. When iodine allergy precludes a contrast-enhanced CT examination, gadolinium may be administered without cross-reactivity. Additionally, when all IV contrast agents are contraindicated (see below), noncontrast MRA may also provide useful and diagnostic information.
Disadvantages and Limitations of MRI
A major disadvantage to MR is its long acquisition time. A full MR study may take from 30 to 60 minutes. This is not ideal in the cases of acute aortic syndromes, when decisions for intervention are made in a matter of minutes, not hours. Moreover, the long acquisition times may require sedation, particularly in pediatric patients.
In the case of obese patients, the size of the magnet may preclude its use. Magnet size varies by manufacturer and model but, overall, MRI bores are typically narrower than those of CT and may not permit the imaging of obese patients.
Due to the high magnetic fields and tissue heating associated with MR, certain devices are contraindicated. Examples include pacemakers, automated implantable cardiac defibrillators, spinal stimulators, and deep brain stimulators. Metallic fragments in the orbits, brain, or spinal canal may also preclude the use of MRI because fragment heating or motion may result in adjacent tissue damage.
Contrast agents in MRI (gadolinium-based) are excreted through the renal system. Due to the risk of nephrogenic systemic fibrosis, gadolinium agents are contraindicated in those with renal failure. Unlike iodinated contrast, gadolinium remains contraindicated in all cases of renal failure, even when the patient is on hemodialysis.
Problem Solving With Noncontrast MRI
Multiple techniques are used in noncontrast MRI evaluation of the aorta. Black blood sequences (traditionally spin echo sequences) yield high-contrast images of the aortic wall-lumen interface and mediastinal structures. Bright blood (gradient echo) sequences can use both static and cinematic techniques and can yield anatomic and functional data. Also, with the use of velocity-encoded cine sequences, quantitative vascular flow data can be acquired. 3D time-of-flight images can be used for noncontrast angiography. However, time-of-flight images are prone to artifacts (particularly in the setting of slow or turbulent flow) and are less useful when IV contrast can be used.
Problem Solving With Contrast-Enhanced MRI
Following a noncontrast MRI sequence, contrast-enhanced MR images are obtained after the IV administration of gadolinium-based contrast agents. The timing of contrast bolus is critical in the acquisition of MRA sequences because the intravascular phase of contrast enhancement is short.
Contrast-enhanced MRA exploits the T1-shortening characteristics of gadolinium. A postcontrast T1-weighted sequence of the aorta can be obtained using single–breath-hold techniques. Images are obtained in any number of projections and can be reformatted into maximum intensity projection or surface rendered images.
Similar to CT, a noncontrast series of images must first be obtained. In addition to providing a baseline to evaluate relative enhancement, this allows for subtraction images to be calculated from the added information.
■
Aortic Anatomy
Normal Thoracic Aortic Anatomy
The thoracic aorta arises from the left ventricular outflow tract at the aortic valve and extends to the level of the diaphragm ( Fig. 29.1 ). Like all arteries, the aorta is comprised of three layers. The intima is the innermost layer. Aortic media has smooth muscle cells and comprises the middle layer of the aorta. The adventitia is the outermost layer. The adventitia has its own aortic supply, known as the vasa vasorum , which is a network of feeding arteries that encase the aorta. The vasa vasorum can play a role in the development of aortic disease.


The thoracic aorta is divided into three anatomic sections—ascending aorta, aortic arch, and descending aorta. The aortic root is the first portion of the thoracic aorta, between the aortic annulus and the sinotubular junction. The sinuses of Valsalva are considered part of the aortic root and give rise to the coronary arteries. It is important to remember that the first 3 cm of the ascending aorta are within the reflections of the pericardium, an important consideration in the evaluation of ascending aortic disease.
The arch of the aorta starts at the origin of the brachiocephalic artery and extends to the origin of the left subclavian artery ( Fig. 29.2 ). The aortic arch has three branches—brachiocephalic artery, left common carotid artery, and left subclavian artery. The end of the left subclavian artery is at the aortic isthmus, where the left subclavian artery extends to the ligamentum arteriosum.

The descending aorta extends from the isthmus to the diaphragm. Multiple branches arise from the descending aorta, including the bronchial arteries, spinal arteries, intercostal branches, and superior phrenic branches.
Normal Variants of Aortic Anatomy
In 7% of the asymptomatic population, the left vertebral artery arises directly from the aortic arch. This is usually of little clinical significance, but is helpful to know prior to catheter-directed angiography or surgery.
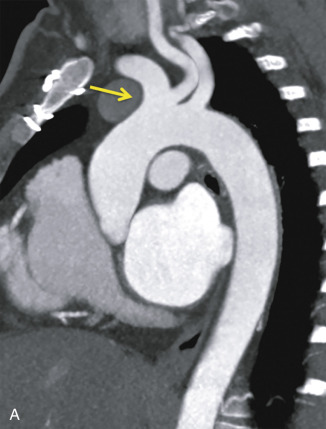
Another common variant to the aortic arch branching is the two-vessel arch, which is characterized by a common origin of the brachiocephalic and left common carotid artery, occurring in up to 25% of the normal population ( Fig. 29.3 ). This anatomic variant has been termed the bovine arch, which is actually a misnomer—a cow’s aortic arch actually has a single brachiocephalic trunk from which the subclavian arteries and a bicarotid trunk arise.

In approximately 0.5% of the population, the right subclavian artery arises directly from the aorta. However, in these cases, the subclavian artery arises distal to the left subclavian origin, taking an aberrant retroesophageal course across the mediastinum.
A ductus diverticulum is also a common aortic arch variant, which occurs at the aortic isthmus. This focal contour abnormality may be complicated by aneurysmal dilation of the diverticulum.
Pseudocoarctation occurs in the setting of an elongated, redundant thoracic aorta. In this case, the redundant portions of the vessel become kinked on themselves. These redundant folds in the aorta can mimic focal narrowing or congenital coarctation of the aorta. Pseudocoarctation is common in patients with Turner syndrome as a result of an elongated transverse portion of the aortic arch.
A cervical aortic arch occurs when the aortic arch extends superiorly beyond the clavicles, into the thoracic inlet. This may be an isolated finding or may occur in the setting of congenital heart and/or thoracic disease.
Pitfalls in the Evaluation of Aortic Anatomy
Pseudocoarctation Versus Coarctation
A true coarctation is a congenital narrowing of the aorta, which occurs at the aortic isthmus (at the level of the ductus arteriosus). Thus a true coarctation results in obstruction of flow. Secondary signs of coarctation include left ventricular hypertrophy or enlarged collateral vessels, such as the intercostal and internal mammary arteries.
In contrast, pseudocoarctation results from redundancy of an ectatic aorta. Redundant folds in the aorta can give the appearance of luminal narrowing, although the overall flow in the aorta is not actually obstructed. As such, there should be no collateral vessels and normal flow on phase contrast MRI.
■
Chronic Diseases of the Aorta
Atherosclerosis
Causes of Atherosclerosis
Atherosclerosis is the result of chronic inflammation in an artery secondary to long-standing hypertension. Lipids are deposited into the vessel wall, and inflammatory cytokines and cells infiltrate the vessel wall. A contained atheromatous plaque has a fibrous cap, which protects the lipid core from exposure to the vessel lumen. A plaque may be calcified or noncalcified.
Appearance of Atherosclerosis
Radiographs are relatively insensitive for the detection of noncalcified atheromatous plaque. However, calcified atherosclerosis may be visible in the aortic arch (on a frontal projection) or in the aortic wall (on lateral views). Noncontrast CT is very sensitive for the detection of calcified atherosclerosis; however, noncalcified plaque may not be visible. In contrast, CTA is very sensitive for the detection of noncalcified plaque, whereas calcifications may occasionally be obscured.
MRA is very sensitive for the detection of atherosclerosis, namely as an irregularity along the aortic vessel wall. Calcifications, on the other hand, may be difficult to detect on MRI.
Thoracic Aortic Aneurysm
Normal Dimensions of the Aorta
Problem Solving: Measuring the Aorta
The normal aorta is a curved 3D structure. Thus measurement of the aortic lumen in standard axial, sagittal, and coronal planes may underestimate or overestimate the actual dimensions of the vessel. Thin-section data and interactive reformatting software are essential for the accurate and reproducible measurement of the aorta. Interactive software allows for true reconstruction in the plane of the aorta as it turns and results in accurate cross-sectional analysis of the aorta. This is important not only in the evaluation of acute aortic disease, but also in the surveillance of aortic disease (e.g., evaluating an aortic aneurysm for increasing size). By convention, aortic size is measured from the outermost portion of the aortic wall (outer diameter).
Appearance of a Thoracic Aortic Aneurysm
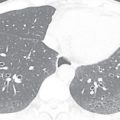
By definition, an aortic aneurysm occurs when all three layers of the aorta (intima, media, and adventitia) become dilated. Although size criteria have been debated, the generally accepted cutoff for normal aortic size is 4 cm ( Fig. 29.4 ).


On a radiograph, this manifests as a large, ectatic, and tortuous aorta. However, artifacts related to projection and multiple overlapping mediastinal structures make measurements of the aorta unreliable by radiographic appearance only. This is better evaluated with multiplanar reformats on cross-sectional imaging, such as contrast-enhanced CT or MRI.
Most thoracic aortic aneurysms (60%) occur in the ascending aorta. The descending thoracic aorta is affected in 40% of cases.
Clinical Significance of a Thoracic Aortic Aneurysm
Due to altered hemodynamics and decreased tensile strength of the aorta, the aneurysmal aorta is inherently weak. The aneurysmal aorta has a higher likelihood of aortic dissection and/or rupture. The risk factors predicting aneurysm rupture are overall size and rate of enlargement. As the aortic size increases, the tensile strength of the aorta decreases, thus increasing the likelihood of rupture.
■
Acute Aortic Syndromes
Intramural Hematoma
Causes of Intramural Hematoma
An IMH is an acute aortic syndrome characterized by acute hemorrhage into the aortic media. It is thought to result from rupture of the vasa vasorum into the vessel wall. An IMH may have an intact intima, although complicated cases may have focal aortic dissection or intimal interruption.
The most common predisposing factor for IMH is hypertension. IMH may also occur because of trauma or a PAU.
Appearance of Intramural Hematoma
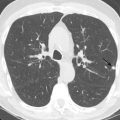
IMH is best characterized on noncontrast CT. On axial images, IMH appears as a crescentic density in the wall of the aorta ( Fig. 29.5 ). The IMH is hyperdense and has different MRI signal characteristics relative to aortic blood pool as the hematoma organizes and becomes thrombosed ( Fig. 29.6 ).