Introduction
CT has become an indispensable part of diagnostic imaging in recent times. With the help of the money that the Beatles’ records made for Electrical and Music Industries (EMI), Nobel Laureate Sir Godfrey Hounsfield invented CT in 1972, a technology that has evolved at a rapid pace since its introduction. The first CT scanner was an axial CT scanner, which took around 5 minutes for the acquisition of each image. Subsequent research in the early to mid-1970s led to the development of four generations of step-and-shoot CT scanners. The third generation of CT scanners, which used a cone-shaped x-ray beam and a rotating x-ray tube–detector assembly, has come to be the most widely used CT scanner design as of now. The major limitation of these axial CT scanners was their lack of temporal resolution and inability to generate volume images. The second phase of technologic overhaul for CT happened in 1989, with the introduction of slip ring technology, which enabled a continuous helical mode of volumetric CT data acquisition without the need for stopping the scan acquisition to uncoil the detector array or the x-ray tube. The introduction of single-detector row helical CT (SDCT) scanners virtually led to a revival of interest in CT in the 1990s.
The most recent wave of revolutionary change in CT technology started in the late 1990s with the introduction of multidetector-row CT (MDCT), which expanded the z-coverage and immensely enhanced the speed and z-resolution (ability to acquire thin-section images over the entire scan range). With multiple additions of detector rows to the MDCT, it is now possible to image long body regions in a matter of a few seconds. Modern MDCT scanners can allow up to 16-cm detector coverage in one x-ray tube rotation around the patient in under 0.4 second. Amid the rush to add rows to the detector array systems, some vendors also have added an additional x-ray tube and detector array panel, which led to the rise of dual-source MDCT technology; the impact on temporal resolution and dual-kilovoltage (kV) scanning has been revolutionary. Growth in CT technology has outpaced all other medical technologies in recent years.
With the increasing use of CT, the radiation dose from CT examinations has become a cause for concern. In 2006, there were an estimated 67 million CT examinations performed in the United States alone. Although CT contributed to only 17% of all radiologic and nuclear medicine procedures, it contributed to around 49% of the collective radiation dose. The National Council for Radiation Protection (NCRP) report 160 stated that the use of CT has increased at a rate of 8% to 15% per year, and there was an increase in effective dose per individual in the United States from 3.6 mSv in the early 1980s to 6.2 mSv in 2006, largely attributable to the increased utilization of radiation-based diagnostic imaging. The radiation doses associated with the same as well as different CT protocols vary widely. For example, the median CT dose index volume (CTDIvol) for pulmonary CT procedures in the United States ranges from 11 mGy for chest CT without intravenous contrast to 26 mGy for CT- guided lung biopsy. On the one hand, modern MDCT scanners enable the evaluation of a larger number of clinical applications at lower radiation doses, which was not possible with older CT systems but, on the other hand, spectacular imaging details and high-power x-ray tubes on modern scanners present the unique risk of overuse and misuse. This suggests the need for tailoring radiation dose protocols to individual patients and indications. In this chapter, we discuss the various options available while designing and tailoring CT protocols in terms of radiation dose.
CT Technology
Key Differences Between Multidetector-Row CT and Single-Detector Row CT Scanners for Chest Imaging
The introduction of multirow detectors has revolutionized CT imaging in many ways. The main advantages of MDCT over SDCT are as follows: (1) higher spatial resolution; (2) faster scanning; (3) ability to scan large volumes; (4) ability to generate superior three-dimensional (3D) reformations; (5) dual-kV scanning modes; and (6) radiation dose efficiency. Spatial resolution is the ability to differentiate between two adjacent points in an image and is measured in pixels. Modern MDCT scanners provide a resolution of up to 1024 × 1024 pixels in contrast to the first scanner designed by Sir Hounsfield, which generated images at a resolution of 80 × 80 pixels. MDCT scanners allow x-ray rotation times as low as 0.25 second, which, coupled with half-rotation reconstruction, enhances the temporal resolution to 0.125 second. With dual-source CT, a quarter-rotation is all that is needed from each x-ray source to generate CT images at a temporal resolution of about 0.063 second.
Acquisition of isotropic image resolution with MDCT allows for the creation of image postprocessing, such as multiplanar reformation (MPR), minimum intensity projection (MinIP), maximum intensity projection (MIP), and volume rendering techniques (VRTs), with exquisite details and without previously noted stairstep artifacts seen with SDCT scanners. These reformats are extremely useful, especially in the study of vascular and complex 3D anatomy.
MDCT also allows for more efficient use of intravenous contrast agents when compared to SDCT. These improvements mean that a chest CT can be completed in less than 1 second on state-of-the-art modern MDCT scanners versus the 15 to 30 seconds needed for SDCT. Such ultrafast scanning abilities at submillimeter resolution along the direction of scanning now allow for the routine generation of excellent 3D images in off-transverse planes. Different MDCT technologies are available to image patients simultaneously at two different levels of kV and generate virtual monoenergetic and material decomposition images while reducing some metal-related artifacts. Fortunately, heightened concerns over radiation dose have also led to significant improvements in radiation dose efficiency for MDCT as compared to SDCT. Modern MDCT scanners come equipped with several hardware and software technologies to enable radiation dose reduction while providing diagnostic image quality.
With the introduction of MDCT, each of the three basic hardware components of CT has advanced considerably. These include the x-ray tube, detector assembly, and data acquisition and processing hardware.
Demands for faster scanning over longer areas of the body with isotropic resolution have been responsible for driving the x-ray tube technology. Modern x-ray tubes now have higher power (100–120 kW) and smaller size. Some of these x-ray tubes can enable the use of 70 to 150 kV, with a maximum tube current of 1300 mA, to enable routine CT scanning at a low kV; this is closer to the k-edge of the iodine and provides better contrast enhancement, with a lower volume of required contrast media. Rapid kV switching on some scanners now allows dual-energy CT (DECT) data acquisition during the same x-ray tube rotation. The use of x-ray beam shaping filters helps carve out the shape of the x-ray beam according to patient size, enabling uniform noise distribution in the image and reducing radiation dose to the thinner peripheral portions of the patient.
Special x-ray beam filters (e.g., tin filter in dual-source CT) at the x-ray source provide better energy separation for DECT on dual-source CT scanners. Selective x-ray beam filtering for a 100-kV x-ray beam using a special tin filter allows for the delivery of a radiation dose as low as 0.1 mSv for lung nodule follow-up CT.
The photon output from an x-ray tube is a continuous spectrum. Although the low-energy photons contribute to the SDCT radiation dose to the patients, they do not contribute to projection generation. The x-ray photons in the scanning plane typically cross an ovoid path. Subsequently, the attenuation around the edges is minimal, and the attenuation is maximal at the center. This leads to an unnecessary radiation dose, as well as artifacts (e.g., beam-hardening artifacts). To minimize the absorption of low-energy photons and beam-hardening artifacts, metal filters (beam-shaping or bowtie shape) are used to shape the x-ray output from the x-ray tube. Bowtie filters are thicker at the periphery than at the center, so that the high-energy photons are filtered out from the periphery, thereby enabling homogeneous image quality as well as minimizing radiation dose. One disadvantage of using filters is that there is some loss of medium-energy photons, which might actually contribute to projection generation. Users must remember that appropriate functioning of these bowtie filters requires good patient centering. Some vendors now provide selectable filtration levels, whereby users can choose the degree of filtration as dictated by clinical needs. Bowtie filters are integral parts of most modern CT scanners.
Key Features of Current State-of-the-Art Modern Multidetector CT Scanners
CT technology has been evolving at an extremely rapid pace. Any discussion about state-of-the-art CT scanners now only holds true for an extremely short time because CT vendors are frequently introducing newer and better technologies.
At the time of this writing, the most modern dual-source CT scanner (Siemens SOMATOM Force) allows a tube current of 1300 mA, with an x-ray tube power of 120 kW for each x-ray tube. Such high milliamperage can now enable the routine use of lower kV (70–100 kV) to improve contrast resolution and reduce radiation dose and contrast volume requirements. Most contrast chest CTs can be performed at 70–80 kV on these scanners. In addition, a wider detector configuration and two sets of x-ray tubes and detector arrays enable the use of a high nonoverlapping pitch (1.6–3.4 : 1); this enables an ultrafast scanning mode in which the entire chest can be scanned in a fraction of a second.
Some other vendors have developed wide-area detectors (e.g., GE Revolution, Toshiba Aquilion One), with 256 to 320 detector rows along the scanning direction. These scanners allow 16-cm coverage in one gantry rotation. Most CT equipment now allows a gantry speed of well under 0.4 second, further shortening scan duration. Faster data acquisition systems with enhanced temporal resolution allow for near–real-time imaging (four-dimensional [4D] imaging).
To reduce radiation dose, most MDCT scanners now come equipped with x-ray beam collimation to improve radiation dose efficiency, which is the ratio of used x-rays falling on the detectors to the x-rays falling beyond the detectors. Postpatient 3D scatter grids help prevent scattered x-ray photons from reaching the detectors and thus help reduce the image noise, which also allows users to reduce the radiation dose. Furthermore, most vendors have introduced competitive iterative reconstruction (IR) techniques for low radiation dose scanning and have replaced traditionally used filtered backprojection (FBP) methods of image reconstruction.
Another vendor (Philips Healthcare) has introduced a multilayered detector to acquire spectral CT data from single-kV datasets. The advantages of such a system are manifold—improved radiation dose efficiency by eliminating the need to acquire simultaneous low- and high-energy acquisitions, ability to achieve a full field of view (FOV) (no disparity in the FOV between the tube-detector assemblies), and elimination of the cross scatter of photons from different tubes.
Modern CT technology is constantly evolving. Discussed in the next sections are a few important hardware features of modern MDCT scanners.
Better Detector Elements
No other component of CT has undergone as many changes in the past decade as the detector elements of CT equipment. With the increasing longitudinal width of detector arrays, coverage per rotation has improved tremendously, as has radiation dose efficiency. Wide-area detector MDCT (≥64 rows) has near-perfect dose efficiency, compared to older four- to eight-detector-row MDCT scanners. Modern MDCT scanners can have up to 256 to 320 detector rows, two detector array panels in a CT gantry (dual-source CT), or two layered detector assemblies (sandwich detector CT). A typical MDCT detector array, which is usually a solid state array, in contrast to the gas-filled detectors used in SDCT, consists of a scintillator, which converts incident x-ray photon flux into light. The latter is converted into an electrical potential by an adjacent layer of photo diode. The electric potential thus generated is amplified and used to generate CT projections using complex mathematical algorithms. Hence, the decay times, as well as the after-glow times of the detector elements, are major determinants of the temporal resolution of a CT scanner. The detector element most commonly used in modern MDCT scanners uses gadolinium oxide, with a ceramic material. This assembly has after-glow and decay times in the range of a few microseconds, which allow for faster acquisition of projection data and, in dual-energy scanners, rapid kV switching.
Prepatient Dose Efficiency
There are two factors that could affect CT radiation dose efficiency (see earlier). Overbeaming refers to the part of the x-ray beam that falls beyond the detector rows and cannot be used for image reconstruction. Modern MDCT scanners use real-time collimator cams to focus the x-ray beam on the detectors and reduce the extent of overbeaming.
Overranging refers to the portion of x-ray beam at the start and end locations of helical CT acquisition. Many contemporary MDCT scanners have prepatient adaptive shield collimators to reduce the extent of overranging at the start and end of a helical acquisition.
Scan Factors
Factors That Affect In-Plane Spatial Resolution in CT Imaging
The factors that affect in-plane spatial resolution in CT scanning can be classified as those acting during image acquisition and those acting during image processing. Configurations of the focal spot, detectors, scan FOV, and section thickness are a few image acquisition parameters that affect the resolution. Because CT images are generated by complex mathematical interpolation and calculation techniques, the algorithms used to generate images significantly affect image resolution. The pixel size of an image, which is determined by the scan FOV and matrix size, is given by this formula:
Pixel size=Scan FOV/(Matrix size)
In general, a smaller FOV provides better spatial resolution but may not provide skin-to-skin coverage in larger patients. Thus routine chest CT may be limited to FOV spanning from skin to skin as much as possible. However, for specific protocols, such as workup of the aorta or diffuse lung disease, spatial resolution of images should be enhanced with the use of a smaller FOV. For example, rib-to-rib coverage with a smaller FOV is sufficient for a diffuse lung disease protocol and helps improve the in-plane spatial resolution.
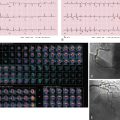
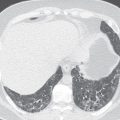
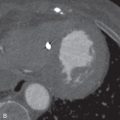
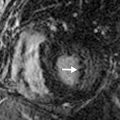
Section thickness or slice width in SDCT is determined by the width of the x-ray beam, whereas in MDCT, nominal slice width is determined by the configuration of the detector, rather than the x-ray beam width. The width of detector rows (≥32 detector rows) in modern MDCT scanners varies among different vendors and is usually about 0.5 to 0.625.mm ( Figs. 6.1 and 6.2 ). One advantage to using multiple rows of detectors is that it allows the user to choose the nominal slice width. For example, let us assume that an MDCT scanner has an effective detector row width of 0.625 mm and a total of 40 detector rows. These rows could be adapted to attain a nominal slice width of 0.625, 1.25, 3.75, or 5 mm, depending on user preference and clinical indications. An important caveat to this is in the older generation of MDCT scanners (≤16 years), where variable thickness detector rows necessitate a priori specification of the required slice width. For example, on a 16-MDCT scanner, if a detector configuration of 16 × 1.25 mm is chosen for scanning, only 1.25-mm and wider slices can be generated, and 0.625-mm sections cannot be generated (will require use of a 16 × 0.625 mm configuration). For diffuse lung disease, it is desirable to obtain a section thickness of 1 mm or less, whereas most other chest indications can be imaged at a 1.25-mm slice width or more. Attaining isotropic resolution was a challenge in SDCT scanners. However, the detector configuration in modern MDCT scanners allows for thinner collimation, which in turn enhances the resolution of images in the z-axis. This has led to reduced stair-stepping artifacts and enhanced image resolution. Thin collimation also reduces partial volume effects by reducing voxel size. Despite increases in image noise with thin collimation (if the tube current–time product is held constant), thin sections have better contrast and spatial resolution.








Factors Affecting Scan Time
Wider x-ray beam or detector configuration is typically more dose-efficient compared to smaller detector configuration for covering longer scan volumes, such as the entire chest. If all scanning parameters are held constant, then wider detector configuration will enable quicker scanning across patient length. Thus, a 64- × 0.625-mm detector configuration will enable faster scanning as compared to 32 × 0.625 mm. For regions such as the thorax, where motion artifacts are not uncommon and can negatively affect assessment of the findings, a faster scanning mode is advantageous.
In addition to the detector configuration, beam pitch (ratio of table feed per rotation to the detector configuration) also affects the scan time. A higher nonoverlapping pitch ratio (>1 : 1) allows faster scanning as compared to smaller, overlapping pitch values (<1 : 1) if all other scan parameters such as detector configuration and rotation time are kept constant. For most chest CT examinations, it is preferable to use a pitch ratio of 1 : 1 or higher to enable quicker scanning. On some scanners, use of a lower pitch is associated with a higher radiation dose, whereas on others there is no dose penalty with a change in pitch value. Another caveat is that on some MDCT scanners, the effective slice width is wider than the selected slice width at pitch values greater than 1 : 1. This should be considered specifically when developing a protocol for diffuse lung disease evaluation on these scanners. On some dual-source CT scanners, a very high nonoverlapping pitch ratio (>1.5 : 1) can enable scanning of the entire chest in about 0.5 second. In patients who are unable to hold their breath, such fast scanning mode can be used without breath-holding.
The scan time is also affected by the gantry rotation times. Most modern MDCT scanners allow at least a 0.5-second rotation time. For most chest CT examinations, a shorter scan time is preferred. For very large patients (weight ≥125 kg) on CT scanners with limited mA (especially on some scanners, with ≤16 detector rows), a slower gantry speed (>0.5 second) may be needed to obtain acceptable image quality. CT scanners (generally with ≥16 detector rows) with sufficiently higher tube current abilities usually will not require a slower gantry speed, even in larger patients undergoing chest CT. Some scanners (e.g., Siemens Definition Force) allow half-segment reconstruction at a gantry rotation time of 0.25 second to scan patients who cannot hold their breath.
In summary, pulmonary CT examinations on modern MDCT scanners should be performed in a single breath-hold, with the shortest scan duration, to minimize motion artifacts.
Dual-Energy CT
Introduction
Different tissues may have similar x-ray attenuation characteristics at certain x-ray photon energies. X-ray photons interact with tissues in two different ways: Compton scattering and a photoelectric effect. Intravenous contrast agents are best seen with a photoelectric effect. Photoelectric effect refers to displacement of electrons from the innermost shell of an atom on interaction with x-ray photons of sufficient energy. The displaced electrons are then replaced by electrons from the outer shells, resulting in the production of characteristic radiation. Each element has a particular value of energy (k-edge), which an incident photon must possess to displace the innermost shell electrons. This value (k-edge) depends on the atomic number of the element. X-ray photons interacting with an atom are attenuated maximally if their energy is similar to the k-edge of the atom. X-ray photons produced at 80 kV have energy values close to the k-edge of iodine. Hence, attenuation of photons with iodinated contrast is maximal at 80 kV, which is the lowest allowed value on most CT scanners. However, such a low kV is practical in relatively few patients, given the limitations of maximal tube current constraints in most MDCT scanners. Although low kV imaging may be acceptable in relatively small patients, in larger patients such low-kV scanning will be associated with higher image noise and potential artifacts. These latter constrains often require an increase in tube current, with decreased kV, which may not be feasible or practical with most MDCT scanners.
The use of a dual-energy or dual-kV scanning mode enables near-simultaneous acquisition of a lower and higher kV datasets, which combine high-contrast, high-noise, low-kV information with lower noise and lower artifact information from high-kV scanning. In addition, DECT helps identify subtle differences in attenuation characteristics of tissues at different energy levels of photons. Although the term dual-energy CT indicates that x-rays of only two energy levels are used, in reality the output of x-ray photons from an x-ray tube is a continuous polychromatic spectrum. The tube voltage represents the maximum energy of the photons (kVp = peak voltage), but the mean energy of the photons in the spectrum is usually significantly lower than the peak energy. Subsequently, when two different values of tube voltage are used, the resulting photons have a significant overlap in their spectrum. Most scanners use tube voltages of 80 and 140 kV, although some newer scanners allow the use of 80/90/100 kV with 150 kV. Tin filters of varying thickness have been used to filter out low-energy x-ray output from the higher tube voltage spectrum to reduce the spectral overlap and enhance image contrast. There are a few ways in which DECT data can be acquired; the most commonly used methods are listed in Table 6.1 .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree