Introduction
Hypersensitivity pneumonitis is an immunologically mediated lung disease that occurs in susceptible sensitized individuals following inhalational exposure to antigenic organic dust. As such, the antigenic material must be small enough to deposit in the bronchioles and penetrate the alveoli, where it induces an inflammatory response. The vast majority, around 95%, of patients with hypersensitivity pneumonitis are nonsmokers. It is proposed that this may be due to nicotine impairment of T cell function or decreased antibody production following exposure to inhaled antigens in smokers relative to nonsmokers.
Causes of Hypersensitivity Pneumonitis
A multitude of antigenic materials have been implicated as causative agents in hypersensitivity pneumonitis. The two most studied and described types of hypersensitivity pneumonitis are farmer’s lung and bird fancier’s disease (pigeon breeder’s lung). The first of these, farmer’s lung, is caused by the inhalation of thermophilic actinomycetes species, including Saccharopolyspora rectivirgula (formerly Micropolyspora faeni ) or Thermoactinomyces spp., which grow in moldy hay and grains. Bird fancier’s disease (pigeon breeder’s lung) typically occurs following exposure to birds and is caused by avian proteins found in feathers and excreta. However, this disease has also been shown to occur following the use of down pillows or laundering a pigeon breeder’s clothing. Hypersensitivity pneumonitis has also been described following exposure to air and water systems ( Table 21.1 ) and has been associated with a variety of occupational and recreational exposures ( Table 21.2 ).
| EXPOSURE | CAUSATIVE AGENT |
|---|---|
| Humidifiers | Klebsiella oxytoca, thermophilic actinomycetes |
| Air conditioners | Thermophilic actinomycetes |
| Hot tubs (indoor) | Mycobacterium avium complex, a Cladosporium cladosporioides |
| Indoor swimming pool | Pseudomonas and other gram-negative rods |
a Whether or not the clinical manifestations of Mycobacterium avium complex exposure represents a subset of hypersensitivity pneumonitis or a different but related disease is still a matter of debate.
| OCCUPATIONAL OR RECREATIONAL EXPOSURE | CAUSATIVE AGENT a |
|---|---|
| Maple tree bark | Cryptostroma corticale |
| Mushroom workers | Thermophilic actinomycetes, fungal spores |
| Corn and malt workers | Aspergillus spp. |
| Greenhouse workers and workers with exposure to cheese, dried sausage, cork, or peat moss | Penicillium spp. |
| Automobile industry: painting, parts manufacture or machine operator | Toluene diisocyanate; metal-working fluids ( Mycobacterium avium complex, b Pseudomonas, other gram-negative rods) |
| Foam part production | 1,3-bis(isocyanatomethyl)cyclohexane prepolymer |
| Wood chip boards | Diphenylmethane diisocyanate |
| Bakers | Aspergillus fumigatus and the flour mite Acarus siro |
| Plasterers | Esparto dust |
| Saxophone player | Candida, Ulocladium botrytis , Phoma spp. |
| Recreational painting | Isocyanate |
a Low-molecular-weight chemicals may combine with host proteins to form haptens, which then induce hypersensitivity pneumonitis.
b Whether or not the clinical manifestations of Mycobacterium avium complex exposure represents a subset of hypersensitivity pneumonitis or a different but related disease is still a matter of debate.
Clinical Manifestations
The clinical manifestations of hypersensitivity pneumonitis are highly variable and depend on the frequency, length, and degree of antigenic exposure and on the individual patient’s immune response. Hypersensitivity pneumonitis has been subdivided into acute, subacute, and chronic forms. Although this system has been called into question, this chapter will be presented using these subdivisions, as has been the convention.
Acute hypersensitivity pneumonitis typically occurs 4 to 6 hours following the inhalation of a large quantity of antigenic material and is thought to be mediated by a type III hypersensitivity reaction (immune complex deposition). Clinically, acute hypersensitivity pneumonitis presents with severe dyspnea and constitutional or flulike symptoms (e.g., fever, chills, malaise, myalgias, dry or mildly productive cough, chest tightness) mimicking a viral or bacterial infection. Acute hypersensitivity pneumonitis typically resolves over several hours to a few days following termination of exposure to the antigen. However, it may recur following repeat exposure.
Both subacute and chronic hypersensitivity pneumonitis present insidiously following chronic or intermittent low-level exposure to antigenic material and are thought to be mediated by type IV (cell-mediated) hypersensitivity reactions. Clinically, patients present with progressive dyspnea, chronic cough, fatigue, anorexia, and weight loss and may be confused with malignancy. Subacute hypersensitivity pneumonitis typically resolves following a protracted illness. Chronic hypersensitivity pneumonitis, on the other hand, tends to result in irreversible lung damage. Acute exacerbations can occur at any time, even without further antigenic exposure.
Clinical Characteristics That Suggest the Diagnosis
There is currently no consensus regarding the diagnostic criteria for hypersensitivity pneumonitis, and diagnosis is generally only achieved after multidisciplinary collaboration emphasizing the history and physical examination, laboratory findings, imaging, and/or bronchoalveolar lavage (BAL). Although a confident diagnosis of hypersensitivity pneumonitis can be achieved based on the combination of characteristic imaging findings, presence of exposure to a known inciting antigen, BAL results, and/or identification of serum precipitating antibodies (precipitins), histopathologic examination of transbronchial, open, or video-assisted lung biopsy specimen is sometimes required.
History of exposure is a vital component of the workup of suspected hypersensitivity pneumonitis, and it is imperative that common occupational and recreational causes be explored. An important adjunct to this is serum studies for circulating immune complex and precipitins. Despite these measures, the offending agent may never be elucidated.
BAL can lend substantial support to the diagnosis of hypersensitivity pneumonitis. A normal BAL differential includes <10% lymphocytes. In hypersensitivity pneumonitis, BAL tends to demonstrate lymphocytosis (≥20% lymphocytes in current smokers and ≥30% in never-smokers and former smokers), often >50%. A BAL CD4 + /CD8 + ratio of <1.8 (often <1) is also typical of hypersensitivity pneumonitis.
Histopathologically, all phases of hypersensitivity pneumonitis demonstrate peribronchiolar inflammation. Given the fleeting nature of acute hypersensitivity pneumonitis, biopsy is rarely required or performed in the acute setting. When biopsied, it typically shows neutrophilic peribronchial and bronchiolocentric (near respiratory or terminal bronchioles) and/or alveolar inflammation. Diffuse alveolar damage and temporally uniform, nonspecific, chronic interstitial pneumonitis may also be seen.
Subacute disease typically shows cellular mononuclear (predominantly lymphocyte and plasma cell) infiltration and bronchiolocentric inflammation (near respiratory or terminal bronchioles), peribronchiolar fibrosis, and poorly formed noncaseating interstitial granulomas. Areas of organizing pneumonia may also be seen. In some cases, antigenic material is visible, or organisms may grow on culture.
As in the acute and subacute forms, lymphocytic peribronchiolar inflammation is also seen in chronic hypersensitivity pneumonitis, but with plasma cell predominance. Like the subacute phase, giant cells or granulomas are also seen, and antigenic material may be visible, or organisms may grow on culture. However, the histologic hallmark of chronic hypersensitivity pneumonitis is fibrosis, which may occur in one of three distinct patterns: homogeneous with linear fibrosis and no architectural distortion (nonspecific interstitial pneumonia [NSIP]-like), peripheral subpleural patchy fibrosis and architectural distortion (usual interstitial pneumonia [UIP]-like), and irregular, predominantly peribronchiolar fibrosis (mixed type).
Imaging Findings
The imaging findings of hypersensitivity pneumonitis are highly variable and dependent on the phase of hypersensitivity pneumonitis.
Conventional Radiographic Findings
Chest radiographs in acute hypersensitivity pneumonitis are usually normal; however, patchy or diffuse homogeneous or heterogeneous opacities may be seen. By contrast, radiographs taken during the subacute phase are generally abnormal and typically demonstrate subtle, poorly defined micronodules, with or without reticulation, in a diffuse or mid and lower lung distribution. There may be small heterogeneous opacities but, unlike the acute phase, diffuse homogenous opacities are generally not seen. Chronic hypersensitivity pneumonitis is characterized radiographically by fibrosis consisting of reticulation, architectural distortion, and volume loss. Nodular or ground-glass opacities are occasionally seen, and radiographs are only rarely normal. The findings in chronic hypersensitivity pneumonitis typically predominate in the mid and upper lungs, with relative sparing of the bases.
High-Resolution CT Findings
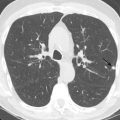
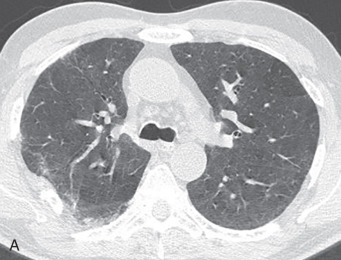
High-resolution CT (HRCT) is much more sensitive to the changes seen in hypersensitivity pneumonitis than radiography. Features that may be seen by CT at any stage of disease include ground-glass opacities, poorly defined centrilobular ground-glass nodules, mosaic attenuation, and air trapping. The combination of normal lung interspersed with ground-glass opacities and low-attenuation foci produces a characteristic pattern called the head cheese sign ( Fig. 21.1 ). The combination of ground-glass opacity or poorly defined centrilobular nodules and air trapping is suggestive of hypersensitivity pneumonitis, regardless of the stage of disease (other disease conditions such as respiratory bronchiolitis–interstitial lung disease [RB-ILD] may also have this appearance).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree