ACR Quality and Safety Programs
Jeffrey P. Kanne
LEARNING OBJECTIVES
1. Describe the quality programs offered by the American College of Radiology (ACR)
2. Understand the purpose behind ACR registries and how participants can improve their respective practices through registry participation
The American College of Radiology (ACR) is a not-for-profit organization that aims “to serve patients and society by maximizing the value of radiology, radiation oncology, interventional radiology, nuclear medicine and medical physics by advancing the science of radiology, improving the quality of patient care, positively influencing the socioeconomics of the practice of radiology, providing continuing education for radiology and allied health professions and conducting research for the future of radiology.”1 ACR has developed various programs to support quality improvement and safety core elements of its mission, many in response to requirements of accrediting bodies and government mandates. These programs include accreditation and other designations, radiology data registries, and peer review.
ACCREDITATION
The Medicare Improvements for Patients and Providers Act (MIPPA) and Mammography Quality Standards Act (MQSA) mandate accreditation of certain imaging modalities. MIPPA requires all private outpatient facilities that offer computed tomography (CT), magnetic resonance imaging (MRI), breast MRI, nuclear medicine, and positron emission tomography (PET) be accredited to bill for technical components of examinations under Part B of the Medicare Physician Fee Schedule. The requirements focus on personnel qualifications (nonphysician medical staff, medical directors, and supervising physicians), image quality, equipment performance, safety standards for both staff and patients, and quality control and quality assurance. MQSA mandates that all facilities providing mammography be accredited.2
ACR offers accreditation programs in various imaging modalities (Table 1.1) and also provides accreditation for radiation oncology through the Radiation Oncology Practice Accreditation Program (ROPA). Other accrediting bodies include The Joint Commission (TJC) and the Intersocietal Accreditation Commission (IAC). After initial accreditation with ACR, facilities must engage in ongoing quality assurance and maintain records of personnel qualifications, as well as apply for renewal to maintain accreditation. To preserve the integrity of accreditation programs, ACR may make unannounced site visits to ensure that facilities continue to meet accreditation requirements after accreditation is granted.
Personnel qualifications for physicians include training, board certification, ongoing experience with interpretation and reporting, and continuing medical education. Radiologic technologists must meet appropriate licensure and continuing education requirements. Medical physicists must meet training or board certification requirements and must document continuing experience with specific equipment surveys and continuing education.
NATIONAL RADIOLOGY DATA REGISTRY
The National Radiology Data Registry (NRDR) is an ACR program that consists of several registries (Table 1.2) and allows participating
facilities to compare their own benchmarks with regional and national peers. Currently, the NRDR consists of the National Oncology PET Registry (NOPR), the CT Colonography Registry, the General Radiology Improvement Database (GRID), the National Mammography Database (NMD), IV Contrast Extravasation Registry (ICE), and the Dose Index Registry (DIR).3 In 2014, the NRDR was designated a Qualified Clinical Data Registry (QCDR) for the Centers for Medicare & Medicaid Services’ (CMS) Physician Quality Reporting System (PQRS).
facilities to compare their own benchmarks with regional and national peers. Currently, the NRDR consists of the National Oncology PET Registry (NOPR), the CT Colonography Registry, the General Radiology Improvement Database (GRID), the National Mammography Database (NMD), IV Contrast Extravasation Registry (ICE), and the Dose Index Registry (DIR).3 In 2014, the NRDR was designated a Qualified Clinical Data Registry (QCDR) for the Centers for Medicare & Medicaid Services’ (CMS) Physician Quality Reporting System (PQRS).
Table 1.1 CURRENTLY AVAILABLE ACR ACCREDITATIONS. | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Table 1.2 REGISTRIES AND DATABASES CURRENTLY PART OF THE NATIONAL RADIOLOGY DATA REGISTRY. | |
|---|---|
|
The DIR enables facilities to compare their CT dose indices with aggregate data from other participating facilities, itemized by body part and exam type (Fig. 1.1). DIR data can be used for the practice quality improvement (PQI) component of the American Board of Radiology (ABR) Maintenance of Certification (MOC) program.4
The CT Colonography Registry tracks both process measures and outcome measures for CT colonography (CTC) (Fig. 1.2). Process measures include optimal bowel cleansing and distention, rate of adequacy of diagnostic CTC exam, and rate of adequacy of screening CTC examination. Outcome measures tracked include rate of colonic perforation, true positive rate, and extracolonic findings.5
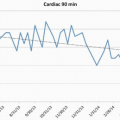
GRID consists of multiple measures related to MRI, patient safety, process, and outcomes (Table 1.3). Current process measures include patient wait time, time from order to exam, reacquisition rate, and report turnaround time. Outcome measures include rates of nondiagnostic liver and lung biopsies, rate of lung biopsies resulting in pneumothorax that require tube drainage, rates of contrast extravasation, and rate of nonconcordant stereotactic breast biopsies.6 Each participating site is provided with a feedback report showing performance and comparison with other GRID sites (Fig. 1.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree