Acute Abdomen in Intensive Care Patients 4 Acute Cholezystitis and Cholangitis Acute (Pyelo)nephritis (Urosepsis) Acute Gastrointestinal Bleeding This chapter reviews the causes and radiologic features of the acute abdomen in intensive care patients. Particular attention is given to the pathologic conditions that may lead to an acute abdomen in the intensive care setting and must be considered in the differential diagnosis of sudden clinical deterioration in intensive care unit (ICU) patients, even if the patient does not or cannot complain of abdominal pain. They include conditions such as (severe) pancreatitis and (acalculous) cholecystitis, acute gastrointestinal bleeding, acute intestinal ischemia, inflammatory bowel diseases with risk of perforation, and pyelonephritis. These complications may arise in both medical and surgical ICU patients. The complications associated with abdominal surgery are discussed in Chapter 5. Acute pancreatitis is characterized by the leakage of pancreatic enzymes from the acinar cells into the adjacent parenchyma and, since the pancreas lacks a capsule, into extrapancreatic tissues. Even today, severe necrotizing pancreatitis has a high mortality rate and requires intensive medical care. The most important imaging modality for evaluating acute pancreatitis is computed tomography (CT). The primary imaging goal is not to make a diagnosis but to assess the severity of the disease and detect complications. The causes of pancreatitis are diverse and range from intrinsic, usually chronic sources of irritation such as alcohol abuse, hypercalcemia, hyperlipidemia, and hepatobiliary stones (biliary pancreatitis) to acute extrinsic causes such as trauma or stress (postoperative), medications, or previous ERCP (endoscopic retrograde cholangiopancreatography). Patients with pancreas divisum are at increased risk for pancreatitis. Various clinical scores have been devised to evaluate the severity and prognosis of pancreatitis (Table 4.1). Severe pancreatitis is indicated by the presence of at least three Ranson criteria identified at the start of the disease or during its course. The mortality rate ranges from 1% (0–2 criteria) to 100% (7 or 8 criteria). Possible associated clinical findings in severe pancreatitis are paralytic ileus (see Chapter 5, p. 164), acute renal failure, or circulatory impairment with tachycardia and hypotension ranging to shock. Plain abdominal radiographs (supine, left lateral decubitus) are useful for excluding “free air” due to a perfora tion and for detecting associated signs (paralytic ileus), although they are of limited value in supine ICU patients. Pancreatic changes are demonstrated more clearly by abdominal ultrasonography, but the results are often compromised by gaseous distension of the bowel. The most important imaging modality for the evaluation of acute pancreatitis is computed tomography (Tables 4.2, 4.3). The goals of CT imaging are to assess the severity of the disease and detect possible complications. CT is not essential for the diagnosis of acute pancreatitis. The plain abdominal radiograph will sometimes show an isolated, gas-distended small bowel loop in the left upper quadrant of the abdomen (sentinel loop sign) or a distended transverse colon segment that terminates abruptly near the inflamed and enlarged pancreas (colon cutoff sign). Isolated distension of the duodenum may also signify an inflammatory process of the pancreatic head (Fig. 4.1a). These signs are neither sensitive nor specific for acute pancreatitis, however. It is more rewarding to look for complications of other diseases in the abdominal cavity (e. g., a perforated duodenal ulcer) or check for associated signs such as paralytic ileus and pleural effusions.
Acute Pancreatitis
M. Uffmann
Pathogenesis
Clinical Aspects
Diagnostic Strategy
Imaging
Plain Abdominal Radiograph
Clinical findings | Time after onset of signs and symptoms |
Lack of clinical responseto conservative therapy | ≥ 72 h |
Suspicion of necrotizing pancreatitis | ≥ 72 h |
Equivocal clinical diagnosis | ≥ 48 h |
Sudden deterioration after initial improvement with suspected complications | Immediate |
Clinically severe pancreatitis (Ranson score > 3) | Immediate |
CTSI | Clinical findings |
3–0 | Lack of improvement or deterioration in response to therapy |
3–0 | At 7–10 days or before discharge to exclude clinically latent complications |
0–2 | CT indicated only if complications are suspected |
CTSI = CT Severity Index (see Table 4.4).
Ultrasonography
Under favorable conditions, abdominal ultrasound scanning can evaluate the parenchymal echogenicity, homogeneity, margins, and size of the pancreas. Acute edematous pancreatitis may be associated with an inhomogeneous, predominantly hypoechoic parenchyma (Fig. 4.1b) accompanied by enlargement of the gland (pancreatic head > 3 cm, rest of pancreas > 2 cm).
Computed Tomography
Classification of the severity of acute pancreatitis is based on morphologic CT changes, clinical findings, and laboratory markers. The goal of CT scanning is to detect parenchymal and extraparenchymal areas of necrosis (Table 4.4).
Acute pancreatitis is basically classified into simple and severe forms. Severe acute pancreatitis is characterized by extensive changes in the peripancreatic fat and hemorrhagic fluid collections in the retroperitoneum.
Serous exudative form. CT scans in the serous exudative form of acute pancreatitis show hypodense, nonenhancing exudates with inflammatory spread along preexisting fascial spaces (anterior pararenal space along the Gerota fascia, along the mesenteric root, along the gastrohepatic, gastrosplenic and gastrocolic ligaments; Fig. 4.2). Enzymatically active exudates may also produce fluid collections in the lesser sac, perirenal spaces, and mediastinum. These exudative fluid collections are sometimes difficult to distinguish from areas of fat necrosis. The latter have less well-defined margins, however, and usually show higher CT attenuation (> 25 HU).
Fig. 4.1 a, b Acute pancreatitis.
a Abdominal radiograph (CT scout view) shows the air-filled duodenal loop splayed open by the enlarged, edematous head of the pancreas (sentinel loop sign).
b Ultrasound image shows a thickened, hypoechoic pancreatic head.
Evaluation of the acute inflammatory process | ||
Grade A | Points = 0 | Normal pancreas |
Grade B | Points = 1 | Intrinsic pancreatic changes, focal or diffuse pancreatic enlargement, slightly heterogeneous parenchyma, small intrapancreatic fluid collections (< 3 cm) |
Grade C | Points = 2 | Same as grade B, but with mild inflammatory changes in peripancreatic tissue |
Grade D | Points = 3 | Peripancreatic inflammatory changes greater than in grade B but limited to fluid collections |
Grade E | Points = 4 | Multiple or widespread extrapancreatic fluid collections or abscess formation |
Degree of pancreatic necrosis | ||
Normal | Points = 0 | No necrosis, normal contrast enhancement of the parenchyma |
Slight | Points = 2 | Necrosis involving < 30% of the pancreas |
Moderate | Points = 4 | Necrosis involving 30–50% of the pancreas |
Extensive | Points = 6 | Necrosis involving > 50% of the pancreas |
Hemorrhagic necrotizing form. The hemorrhagic necrotizing form is characterized by focal, sometimes confluent, areas of pancreatic necrosis. The gland is markedly enlarged, inhomogeneous, and poorly demarcated from peripancreatic tissue. The necrotic areas are hypodense. Parenchymal sequestra may appear isodense, while hemorrhagic portions of the pancreas may be hyperdense (Figs. 4.3, 4.4, 4.5).
The extent of the necrotic areas in the pancreas is best appreciated after contrast administration, as necrosis does not enhance with the rest of the gland.
Bacterial contamination of necrotic areas is common and requires immediate surgical or interventional treatment. Drainage is unnecessary if the necrotic material is solid, but liquefied material can be drained. Proper management requires close cooperation between the radiologist and surgeon, close-interval CT follow-ups, and frequent catheter changes in most patients.
Complications and Intervention
Acute pancreatitis may be complicated by superinfection (suppurative pancreatitis or pancreatic abscess), pseudocyst formation, and vascular complications.
Suppurative form. This is a serious complication characterized by superinfection (usually with intestinal bacteria) and abscess formation (Fig. 4.6) occurring ca. 4 weeks after the onset of inflammation. CT-guided fine-needle biopsy to detect bacterial infection is a valuable aid to further treatment planning (surgery versus CT-guided abscess drainage or possible surgical intervention at a later, less critical time). The suppurative form of pancreatitis generally has a poor prognosis with a high mortality rate.
Pancreatic abscess. Pancreatic abscess cannot always be positively distinguished from an (uninfected) pseudocyst by CT. Both lesions appear as well-defined fluid collections (10–30 HU) with an enhancing wall. The detection of gas inclusions is considered a reliable CT sign of bacterial superinfection when other causes (gastrointestinal fistula, previous percutaneous aspiration) have been excluded.
Fig. 4.2 a–c Acute exudative pancreatitis (three different patients).
a Infiltration of peripancreatic fat.
b Bilateral tracking of exudate along the Gerota fasciae.
c The inflamed pancreas has a spongy edematous structure but still shows homogeneous perfusion. Other findings are ascites and infiltration of the peripancreatic fat.
Fig. 4.3 a–c Necrotizing pancreatitis.
a CT shows extensive necrosis in the pancreatic bed with only fragmentary areas of perfused pancreatic tissue (CT Severity Index 6, see Table 4.4).
b Ascites and extensive spreading of necrosis along the Gerota fascia (grade E).
c Spread of necrosis into the lesser pelvis (CT Severity Index 4, grade E).
Fig. 4.4 a, b Necrotizing pancreatitis.
a With involvement of the perirenal space.
b With abscess formation due to super-infection.
Fig. 4.5 a–c Necrotizing pancreatitis (three different patients, see Table 4.4).
a With focal necrosis (grade B).
b With diffuse necrosis and mild perifocal reaction (CT Severity Index 6, grade B).
c With diffuse necrosis and extrapancreatic spread (CT Severity Index 6, grade D).
Fig. 4.6 a, b Infected necrotizing pancreatitis. CT shows extensive abscess formation, air inclusions, and rim enhancement.
Fig. 4.7 a–c Pseudocysts.
a Perihepatic pseudocyst accompanied by a pseudocyst in the pancreatic bed.
b Giant pseudocyst in the lesser sac.
c Mediastinal spread.
Pseudocysts. Pseudocysts (Fig. 4.7) are a noninfectious complication that may develop approximately 4–6 weeks after the onset of pancreatitis. They are often larger than 6 cm and, when untreated, persist for several weeks. Many (but not all!) pseudocysts do not resolve spontaneously and are drained to prevent complications. The indications for drainage are pain, infection, compression of adjacent organs, diameter greater than 5 cm, and recurrence following aspiration or surgery. Aspiration alone is often insufficient and most pseudocysts require drainage, possibly over a period of months.
Vascular complications. Vascular complications include pseudoaneurysms of the visceral arteries, which develop in ca. 10% of cases. The splenic artery is most commonly affected, followed by the pancreaticoduodenal artery and the gastroduodenal artery. Another vascular complication is stricture formation with associated ischemic effects in dependent organs. Pseudoaneurysms may cause acute, life-threatening hemorrhage (Fig. 4.8). Diffuse hemorrhage may also be encountered (Fig. 4.9). In patients with diffuse thickening of the bowel wall, the differential diagnosis has to include ischemic disease in addition to inflammatory disease (Fig. 4.10).
Fig. 4.8 a, b Splenic artery aneurysm.
a Slight perfusion is noted during the arterial phase.
b Perfusion is increased during the venous phase.
Fig. 4.9 a, b Extensive hemorrhage.
a The hemorrhage probably originates from a branch of the mesenteric vein.
b Layering of sediment in the supine patient.
Fig. 4.10 a–c Necrotizing pancreatitis.
a Extensive extrapancreatic exudation, thrombosis of the splenic vein, and partial thrombosis of the portal vein.
b, c Reactive wall thickening of the small bowel (b) and colon (c).
Acute Cholecystitis and Cholangitis
M. Uffmann
Pathogenesis
Acute cholecystitis. Inflammation of the gallbladder is caused by lithiasis in more than 90% of cases. Cholestasis and bacterial superinfection give rise to a purulent or suppurative cholangitis, which may culminate in a highly acute septic–toxic state with high mortality.
Even in the absence of lithiasis, ICU patients on parenteral nutrition may develop a gallbladder inflammation (acalculous cholecystitis) that may also lead to sepsis.
Cholangitis. The etiology of acute cholangitis is analogous to that of acute cholecystitis and is based on an acute obstruction of the bile duct, rather than the cystic duct, by a stone or other process. The obstructed drainage leads to biliary colic and incites an ascending bacterial cholangitis. The development of rapidly progressive sepsis and shock is among the most serious complications. Multiple biliary, pyogenic liver abscesses may develop as a result of acute suppurative cholangitis.
Imaging
Ultrasonography
Although the detection of gallstones is suggestive of acute cholecystitis, the stones may also be an incidental finding. The most reliable sign of acute cholecystitis is thickening of the gallbladder wall to more than 3 mm, with an average thickness of 9 mm (Fig. 4.11). Ultrasound scanning may demonstrate stratification of the gallbladder wall. Perfusion of the gallbladder fundus on color duplex scans is another sign but is less reliable because color-flow signals may also appear in the normal gallbladder wall. Echogenic areas in the omentum and pericholecystic fat are suggestive signs of inflammation but are rarely detectable.
It should be noted that other diseases are also associated with thickening and stratification of the gallbladder wall and with intraluminal membranes in the absence of acute cholecystitis. They include:
 acute hepatitis
acute hepatitis
 hypalbuminemia in the setting of portal hypertension with ascites
hypalbuminemia in the setting of portal hypertension with ascites
 HIV cholangiopathy
HIV cholangiopathy
 heart failure
heart failure
 renal failure
renal failure
Complicated acute cholecystitis. Definitive signs of complicated acute cholecystitis are gas collections in the gall-bladder wall (emphysematous cholecystitis) and focal hypoechoic areas in the gallbladder wall or bed (pericholecystic abscess).
Acute acalculous cholecystitis. Acute acalculous cholecystitis is frequently difficult to diagnose and is often recognized only by noting the development of complications. Ultrasound scanning shows thickening of the gallbladder wall, which may increase on serial scans. Color duplex examination shows increased mural flow signals indicating hyperemia of the gallbladder wall. A hypoechoic rim in the precholecystic fat and gallbladder bed may signify inflammatory edema.
Acalculous cholecystitis is often associated with gall-bladder hydrops (gallbladder transverse diameter > 4 cm, longitudinal diameter > 10 cm), and this may be the only diagnostic clue (Fig. 4.12a). Thus, acalculous cholecystitis should be considered whenever gallbladder hydrops is found in an ICU patient with sepsis.
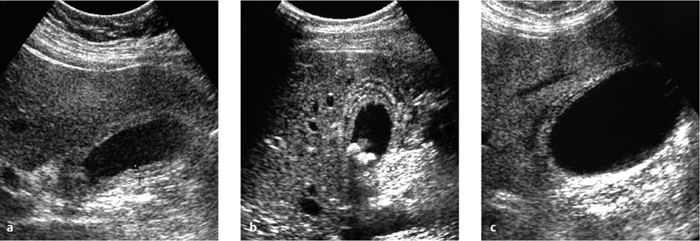
Fig. 4.11 a–c “Typical” ultrasound findings in cholecystitis.
a Inflammatory wall thickening (> 3 mm).
b Stones and wall thickening.
c Three wall layers.
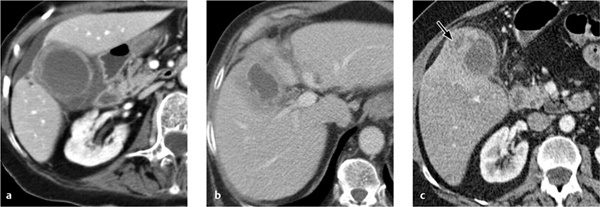
Fig. 4.12 a–c CT findings in cholecystitis.
a Thickened, intensely enhancing gallbladder wall with perihepatic fluid and fluid in the gallbladder bed.
b Incipient pericholecystic abscess formation.
c Confined gallbladder perforation with a frank pericholecystic abscess.
Computed Tomography
CT is generally used in cases where complications are suspected in the setting of cholecystitis (see below). As in ultrasonography, the gallbladder wall is thickened and shows increased contrast enhancement (Fig. 4.12). Usually the adjacent fat is infiltrated owing to pericholecystic inflammation, or fluid may be detected in the gallbladder bed.
Wall thickening and enhancement in the bile ducts (see Fig. 4.14b) is suggestive of cholangitis. Suppuration cannot be detected by analyzing the attenuation values of the biliary fluid.
Complications
The complications of acute cholecystitis include gangrenous or empyematous cholecystitis, gallbladder perforation with the development of a pericholecystic abscess, and gallstone ileus.
Gangrenous Cholecystitis
Gangrenous cholecystitis may develop as a result of gall-bladder distension secondary to an obstruction. This condition will not have serious sequelae if early cholecystectomy is performed.
Sonographic hallmarks of gangrenous cholecystitis are stratification of the gallbladder wall and intraluminal membranes formed by sloughed layers of mucosa. The sonographic Murphy sign (tenderness over the gallbladder) is often negative in these patients.
Pericholecystic Abscess
Untreated, the ischemic wall changes may lead to perforation of the gallbladder followed by the development of a mural or pericholecystic abscess (Fig. 4.12a, b). This may occur even in acalculous cholecystitis. Although a perforation does carry a risk of generalized peritonitis, inflammatory spread at the perforation site is usually contained by omental tissues, resulting in the formation of a circumscribed abscess cavity. Gallbladder perforation may occur, even in the setting of acalculous cholecystitis.
Hypoechoic pericholecystic areas on ultrasound scan are suspicious for abscess formation. With CT the diagnosis of pericholecystic abscess can securely be established by infiltration, possibly accompanied by air inclusions. Neither ultrasonography nor CT can always detect the wall perforation that precedes abscess formation, however.
Emphysematous Cholecystitis
Emphysematous cholecystitis has a relatively high mortality rate (15%). Patients with diabetes mellitus are pre-disposed. Males predominate by a 5:1 ratio. Emphysematous cholecystitis is caused by infection with gas-forming bacteria (Clostridium). Intramural air can typically be detected 24–48 hours after the onset of symptoms. A possible complication is gallbladder perforation with peritonitis.
Diagnosis is based on gas detection in the gallbladder wall by ultrasonography (echogenic foci with acoustic shadowing), CT, and even conventional radiographs in pronounced cases (air–fluid level, Fig. 4.13).
Fig. 4.13 a–c Emphysematous cholecystitis.
a An abnormal elliptical infradiaphragmatic air collection is visible on the portable chest radiograph.
b Ultrasound scan demonstrates echogenic air bubbles in the gallbladder wall.
c CT shows extensive intramural air inclusions, air in the common bile duct, and perihepatic ascites.
Gallstone Ileus
In rare cases the inflamed gallbladder may perforate into the duodenum, resulting in gallstone ileus. Stones larger than 3 cm may become impacted, usually at the ileocecal valve, causing a mechanical bowel obstruction (see Chapter 5, p. 164).
Hepatic Abscess
Hepatic abscess is a potential complication of ascending bacterial cholangitis (Fig. 4.14). Other causes of hepatic abscess are as follows:
 hematogenous spread via the portal vein in intra-abdominal sepsis (appendicitis, diverticulitis), pyelophlebitic abscess
hematogenous spread via the portal vein in intra-abdominal sepsis (appendicitis, diverticulitis), pyelophlebitic abscess
 hematogenous spread via the hepatic artery in sepsis or bacteremia
hematogenous spread via the hepatic artery in sepsis or bacteremia
 spread by local extension (Fig. 4.15)
spread by local extension (Fig. 4.15)
Regardless of the etiology, there are three stages of abscess formation (Table 4.5), which are distinguishable by their morphologic features. Differential diagnosis is often difficult with ultrasonography, and CT can provide a more accurate classification.
Hepatic abscess appears on CT scans as a rounded or irregular area of low attenuation (0–45 HU). The center of the mass does not enhance after contrast administration. The abscess may appear initially as a cluster of small cystic lesions that coalesce over time.
Typical rim enhancement is seen only after a mature abscess membrane has formed. But granulation tissue takes 2–4 weeks to develop, so the absence of typical peripheral enhancement does not exclude an hepatic abscess (Fig. 4.15c).
Contrast enhancement of the granulation tissue may be masked during the portal venous phase by enhancement of the normal liver parenchyma, or the granulation tissue may appear hypodense. The ill-defined appearance of the abscess margins before and after contrast administration is an important differentiating feature.
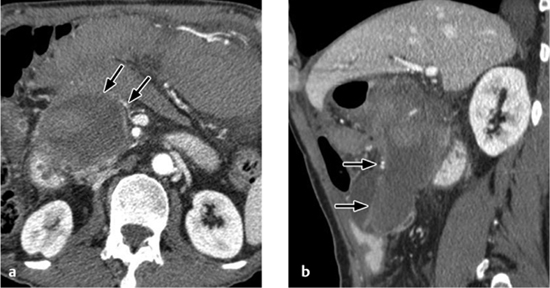
Fig. 4.14 a, b Biliary liver abscess. CT reveals an intrahepatic abscess with air inclusions (a) in cholangitis (b) with dilated intra- and extrahepatic bile ducts and increased enhancement of the duct walls.
Fig. 4.15 a–c Intrahepatic abscesses.
a On ultrasound scans intrahepatic abscesses are often hypoechoic.
b, c On CT the abscesses may be multilobular with increased peripheral enhancement (b) or may have a cystlike appearance (c).
Stage | Time | Features |
Necrosis | 0–10 days | Incipient parenchymal necrosis, small fluid areas |
Reabsorption | 10–15 days | Reabsorption of the necrosis with liquefaction |
Mature abscess | > 15 days | Abscess encapsulated by a fibrous wall |
Besides enhancement of the abscess membrane, IV contrast administration may also produce a ring pattern of contrast enhancement in the adjacent healthy liver parenchyma. This “double-target sign” is caused by increased capillary permeability. Gas collections within an abscess are specific for infection with gas-forming bacteria, but they are very rarely observed.
Acute (Pyelo)nephritis (Urosepsis)
G. Heinz-Peer
Acute pyelonephritis is an infectious nephritis that involves the renal pelvis. In ICU patients with sepsis, pyelonephritis is one possible focus (urosepsis, see section on Sepsis in Chapter 5, p. 154). A diffuse interstitial form is distinguished from a focal form (acute focal bacterial nephritis).
Emphysematous pyelonephritis. Emphysematous pyelonephritis is a particularly severe form of bacterial pyelonephritis caused by gas-forming bacteria. It is common in diabetics (90% of all cases) and is an indication for immediate surgery.
Pyonephrosis. Pyonephrosis results from the infection of a hydronephrotic kidney, and is often associated with an obstructing stone and renal function impairment.
Renal abscess. An intrarenal or perirenal abscess is a complication of infectious nephritis, pyelonephritis, or septicemia. It may result from an ascending infection (gram-negative bacteria) or hematogenous dissemination (Staphylococcus aureus).
The hematogenous spread of infection (usually in staphylococcal sepsis) incites the formation of multiple small renal abscesses. In contrast to ascending pyelonephritis, they are located predominantly in the well-perfused renal cortex. These cortical foci facilitate the spread of infection to the perirenal space.
Diagnostic Strategy
The diagnosis of nephritis and pyelonephritis is based on laboratory serology and clinical findings. Ultrasonography can be helpful in evaluating the degree of obstruction. CT is used to investigate suspected complications (intrarenal or perirenal abscess) and to determine pathogenesis. The possibility of urosepsis with inflammatory renal changes should always be considered when looking for an infectious focus in ICU patients with sepsis.
CT is also used acutely in pyonephrosis to determine the location of the causal obstruction.
Imaging
Sonography
Acute pyelonephritis. Acute pyelonephritis is a clinical and serologic diagnosis. Ultrasonographic findings are usually normal. Rarely, the ultrasound scan may show an enlarged kidney with a bulky, hypoechoic parenchyma and effacement of the sinus echo complex.
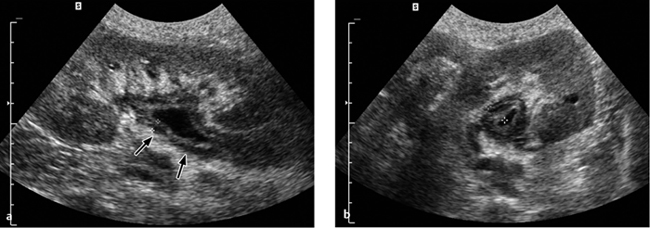
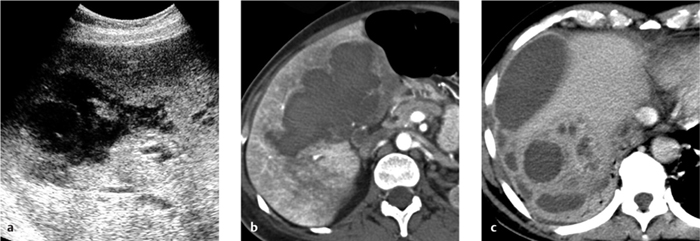
Pyonephrosis. Pyonephrosis, unlike hydronephrosis, has no pathognomonic sonographic findings. The fluid collection may show increased echogenicity due to echogenic aggregates of cells (pus), but this is not consistently seen. The echogenicity may be so high that it creates a tumor-like appearance with complete obliteration of the central echo complex. As in CT, ultrasonography may show marked thickening of the renal pelvic wall in some cases (Fig. 4.16; see also Fig. 4.18). Abscesses and areas of liquefaction may also have a cystlike appearance. Ultrasonically or CT-guided fine-needle aspiration should confirm the diagnosis prior to drainage.
Computed Tomography
Acute pyelonephritis. Acute pyelonephritis leads to focal or diffuse enlargement of the kidney. Focal or wedge-shaped areas of increased attenuation on unenhanced CT may signify hemorrhage, while areas of decreased attenuation indicate edema or necrosis.
Affected areas of the renal parenchyma show decreased contrast enhancement during the parenchymal phase, sometimes forming a radial pattern that extends past the corticomedullary junction. The walls of the renal pelvis and ureter may be thickened and show increased enhancement (see Fig. 4.18).
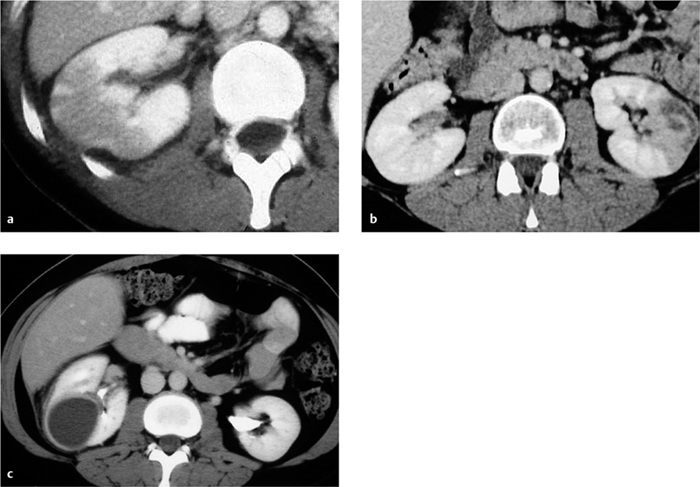
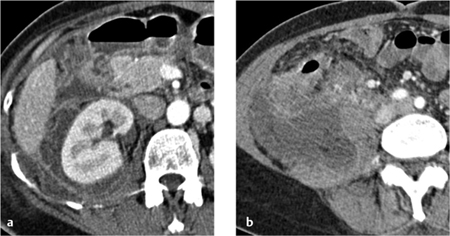
Focal bacterial nephritis. Focal bacterial nephritis (focal pyelonephritis, Fig. 4.17a) has a tumorlike appearance with a focal, ill-defined zone of decreased contrast enhancement. Again, a radial pattern may be seen in the late phase of enhancement, and lack of enhancement indicates necrosis or abscess formation. A focal abscess (Fig. 4.17b) requires differentiation from an infected cyst (Fig. 4.17c).
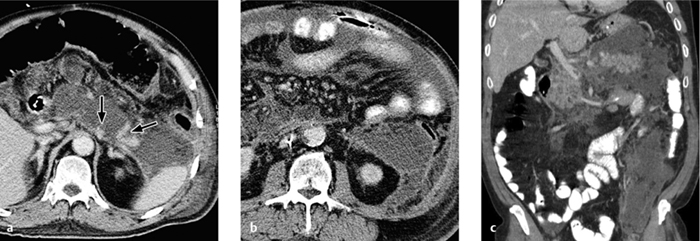
Renal abscess. A renal abscess appears on CT as a mass with a hypodense center and enhancing rim (Figs. 4.18, 4.19). Its attenuation values (10–30 HU) are higher than an uncomplicated cyst, and peripheral enhancement is not invariably present. Fungal infections tend to cause microabscesses, obstruction, and calcifications. Renal abscess cannot always be distinguished from a tumor with central necrosis.
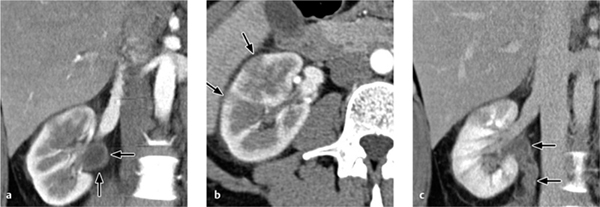
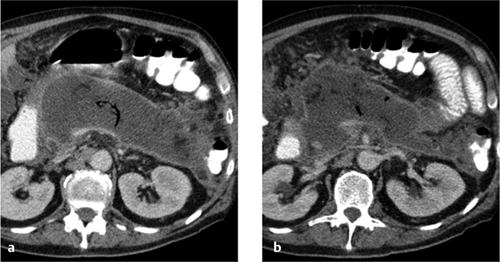
Emphysematous pyelonephritis. Emphysematous pyelonephritis is characterized by the presence of gas in the renal parenchyma and collecting system as well as at parapelvic, subcapsular, and retroperitoneal sites (Fig. 4.20).
Pyonephrosis. The wall of the renal pelvis in pyonephrosis is thickened and shows increased contrast enhancement. Layering of (opacified) urine and pus is found in the dilated renal pelvis, the intrapelvic fluid showing attenuation values > 20 HU (pus). Rare cases show retroperitoneal or even intraperitoneal spread of infection with the development of peritonitis (Fig. 4.21).
The CT changes may persist for months despite appropriate and successful treatment.
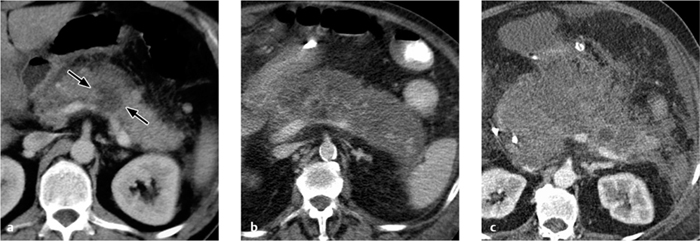
Fig. 4.16 a, b Acute pyelonephritis.
The walls of the renal pelvis and ureter are thickened in a patient with acute pyelonephritis (see also Fig. 4.18).
Fig. 4.17 a–c Complications.
a, b Focal hypodensities in the renal parenchyma are consistent with focal nephritis (a) or frank abscess formation (b)as a complication of infection.
c A cyst with a thickened, enhancing wall is typical in infections.
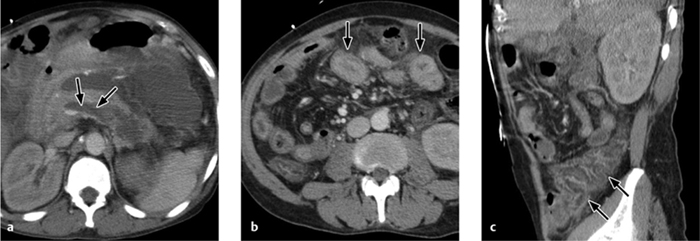
Fig. 4.18 a–c Pyelonephritis with an abscess.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 The main goals of imaging in acute pancreatitis are the assessment of severity and the detection of complications.
The main goals of imaging in acute pancreatitis are the assessment of severity and the detection of complications.
 CT is the principal imaging study for acute pancreatitis.
CT is the principal imaging study for acute pancreatitis. The CT Severity Index is used to evaluate and quantify the inflammatory process and the degree of pancreatic necrosis.
The CT Severity Index is used to evaluate and quantify the inflammatory process and the degree of pancreatic necrosis.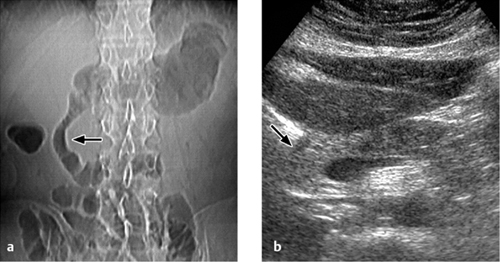
 Viable pancreatic tissue enhances on CT while necrotic parenchyma does not.
Viable pancreatic tissue enhances on CT while necrotic parenchyma does not.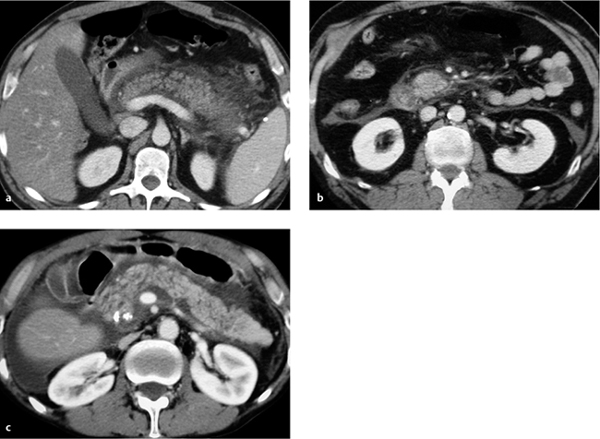
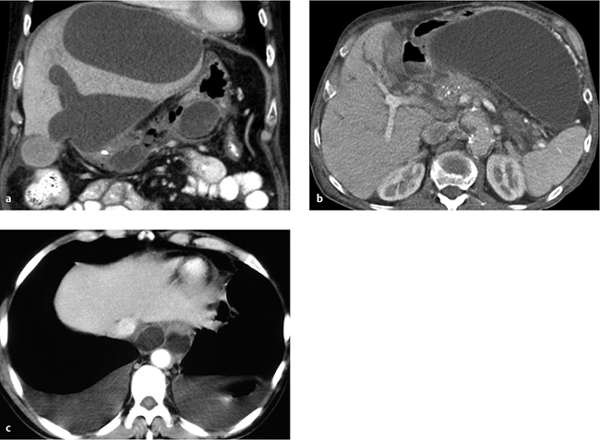
 Pancreatic abscess and pseudocyst are not always distinguishable by CT. Gas inclusions of unknown cause signify bacterial superinfection.
Pancreatic abscess and pseudocyst are not always distinguishable by CT. Gas inclusions of unknown cause signify bacterial superinfection.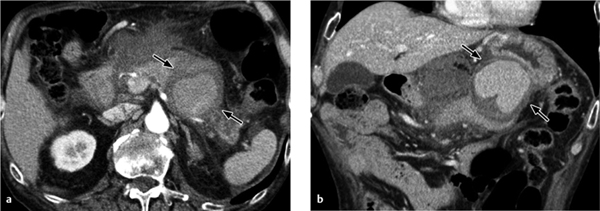
 Gallbladder hydrops in an ICU patient with sepsis should raise suspicion of acalculous cholecystitis, even in the absence of wall thickening and perifocal fluid.
Gallbladder hydrops in an ICU patient with sepsis should raise suspicion of acalculous cholecystitis, even in the absence of wall thickening and perifocal fluid.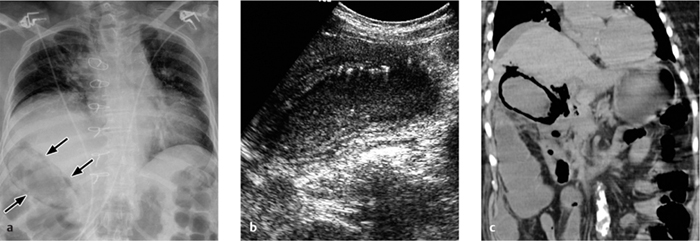
 As it takes 2–4 weeks for an abscess to form a membrane, the absence of typical rim enhancement does not exclude a liver abscess.
As it takes 2–4 weeks for an abscess to form a membrane, the absence of typical rim enhancement does not exclude a liver abscess.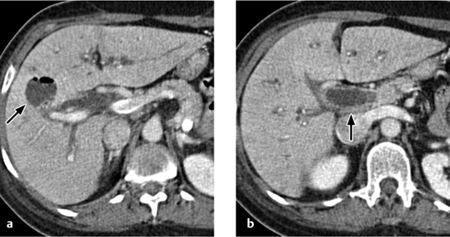
 CT is used acutely in pyelonephritis to investigate suspected complications and is used electively to determine the cause. Always consider the possibility of urosepsis in ICU patients with sepsis!
CT is used acutely in pyelonephritis to investigate suspected complications and is used electively to determine the cause. Always consider the possibility of urosepsis in ICU patients with sepsis!