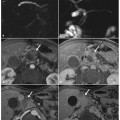
Fig. 6.1
Biliary non-severe acute pancreatitis, with stones within the lumen of the common bile duct at the level of ampulla of Vater. Axial (a) and multiplanar reformatted paracoronal computed tomography (b) images show enlargement of the pancreatic head, with calcifications in the head (a). c–e Axial T2-weighted half-Fourier acquisition single- shot turbo-spin-echo (HASTE) images show gallstones, slightly dilated pancreatic duct in the pancreas head, and enlarged common bile duct, with fluid collection around the gallbladder and edematous stranding of peri-pancreatic fat. f A magnetic resonance cholangiopancreatography image shows a filling defect in the ampulla of Vater, and dilatation of the pancreatic duct and common bile duct
In non-severe acute pancreatitis, MRCP findings are usually normal, and they can include: intraluminal filling defects (stones), in both the gallbladder and the common bile duct; and diffuse stretching or mild ectasia of the main pancreatic duct.
6.4.2 Severe Acute Pancreatitis: MRI Findings
The MRI findings of severe forms of acute pancreatitis (Balthazar grades D and E) are (Fig. 6.2): the same findings as for non-severe acute pancreatitis; stranding of peri-pancreatic fat, which is best seen on in-phase T1-weighted images; peri-pancreatic fluid collections, which are best seen on T2-weighted images and are typically located around the pancreas and in the anterior pararenal space; pancreatic necrosis, for which it is important to determine the extent because it has a significant correlation with prognosis. Necrotic tissue does not enhance; therefore, pancreatic necrosis is seen as diminished or absent parenchymal enhancement after gadolinium administration. Necrosis may not become apparent until 24–48 h after onset of the initial symptoms [16–19], and there is ill-defined phlegmon showing heterogenous content and mixed signal intensity on T2-weighted images (hyperintense and hypointense portions).
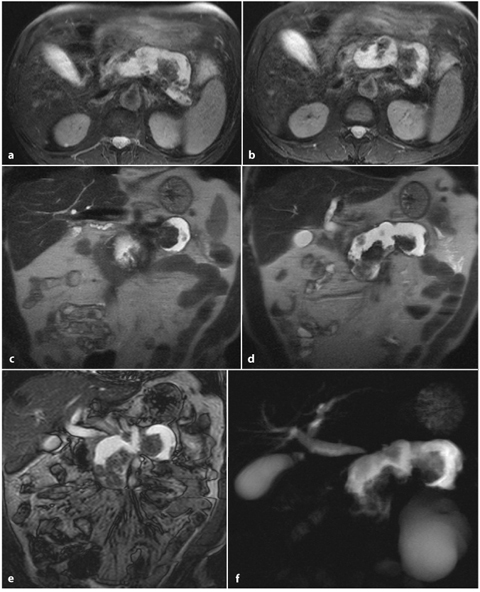

Fig. 6.2
Severe acute pancreatitis, with pancreatic necrosis involving more than 50% of the gland. Axial (a, b) and coronal (c, d) T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) images, and a coronal outof- phase image (e), show a large fluid collection with an inhomogenous hyperintense content with hypointense areas, attributable to necrotic debris in the corresponding areas of the pancreatic body and tail. e Enlargement of pancreatic head. f Magnetic resonance cholangiopancreatography shows the huge necrotic area, with deletion of the main pancreatic duct and common bile duct dilatation
6.4.3 Severe Acute Pancreatitis: MRCP Findings
The MRCP findings of severe forms of acute pancreatitis (Balthazar grades D and E) are (Fig. 6.2): similar ductal changes to those seen in non-severe acute pancreatitis; focal interruption of the pancreatic duct; the main pancreatic duct can be obscured by overlying fluid [15, 20–23].
Table 6.1 shows the MRSI for acute pancreatitis in correlation with Balthazar grade, with typical MRCP appearance [2, 14, 18].
Table 6.1
Acute pancreatitis: magnetic resonance imaging pattern and signal intensity
Balthazar grade | MRI | Fat-saturated T1-weighted image | T2-weighted image | MRCP | Score |
|---|---|---|---|---|---|
A | Normal pancreas | Homogenous, and slightly hyperintense compared with normal liver parenchyma | Homogenous, and slightly hypointense to isointense compared with normal liver parenchyma | Thin main pancreatic duct (normal size 2mm); small secondary pancreatic ducts are not always visible | 0 |
B | Heterongenous, and/or enlarged pancreas | Enlarged, heterogenous, isointense to slightly hypointense compared with normal liver parenchyma | Enlarged, heterogenous, mild hyperintensity | Very thin main pancreatic duct because of the compression of pancreatic edematous parenchyma | 1 |
C | Peri-pancreatic changes | As described above, plus peri-pancreatic fat stranding | As described above, plus peri-pancreatic hyperintnesity and fat stranding | Dilated main pancreatic duct and common bile duct caused by the presence of stones in cholecyst or in the bile tree. | 2 |
D | Peri-pancreatic collection in a sinlge location | Homogenous or heterogenous, confluent, ill-defined, unencapsulated, peri-pancreatic collection | Homogenous or heterogenous, confluent, ill-defined, unencapsulated, peri-pancreatic collection | Ill-defined unencapsulated peri-pancreatic collection, with or without communication with the dilated main pancreatic duct | 3 |
E | Two or more peri-pancreatic collections, or retroperitoneal air | Multiple collections, as described above | Multiple collections, as described above | Multiple collections and rupture of main pancreatic duct, as described above | 4 |
Percentage necrosis: | |||||
None | 0 | ||||
30% | 2 | ||||
30–50% | 4 | ||||
>50% | 6 |
6.5 Pancreatic Necrosis
Severe acute pancreatitis occurs in approximately 20–30% of cases of pancreatitis, and it is usually associated with pancreatic necrosis, increased complications and mortality. Necrosis may develop 48-72 h after the onset of acute pancreatitis, and therefore may not be detected on the initial contrast-enhanced study [24].
Detection and quantification of necrosis is crucially important because it has important prognosis implications. Necrosis involving less than 30% of the pancreatic parenchyma is associated with a low mortality rate, but the presence of more than 50% pancreatic parenchyma involvement raises the mortality rate to 10–25%. It is also important to consider that necrotic tissue can become infected, in which case the mortality rate can rise to 60% (Fig. 6.3) [19].
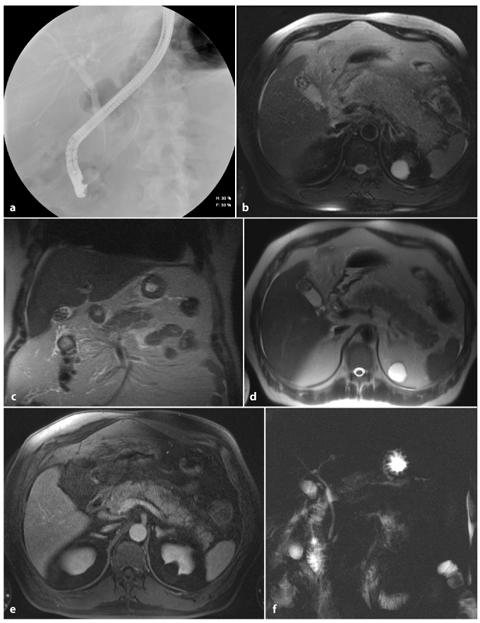

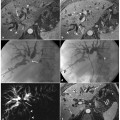
Fig. 6.3
Acute pancreatitis, with focal necrosis of the pancreatic parenchyma less than 50%. a An endoscopic retrograde cholangiopancreatography image shows a normal pancreatic duct and a normal common bile duct. Axial and coronal TSE T2-weighted (b, c) and axial T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) (d) images show enlargement of the pancreatic body and tail, with edematous stranding of peri-pancreatic fat and the presence of gallstones. e A T1-weighted volumetric interpolated breath-hold examination (VIBE) image obtained in the arterial phase shows a decreased focal enhancement in the pancreatic parenchyma. f The magnetic resonance cholangiopancreatography examination was severely limited by overlying liquid artifacts caused by the abdominal effusion
Typical MRI features of pancreatic necrosis are: focal loss of the normal parenchymal acinar pattern on T1-weighted and T2-weighted image sequences, with associated low-signal- intensity areas on T2-weighted images; necrosis can show low signal intensity or, when liquefied, high signal intensity, but it has no specific features on unenhanced scans; in post-gadolinium images, necrotic tissue does not enhance, in contrast to viable tissue. It is fundamental to inject the contrast agent at a rapid rate, and to use adequate bolus timing for accurate detection [18]. The importance of MRCP in pancreatic necrosis is in establishing focal interruption of pancreatic duct.
6.5.1 Differentiation Between Pancreatic Necrosis and Peripancreatic Fluid Collection
In the setting of non-severe acute pancreatitis, the sensitivity of MRI can exceed that of CT, with T2-weighted images being most useful for revealing pancreatic signal changes and peri-pancreatic edema [14], although some authors have reported that non-severe acute pancreatitis is not necessarily depicted by this imaging technique (Fig. 6.4) [25, 26].
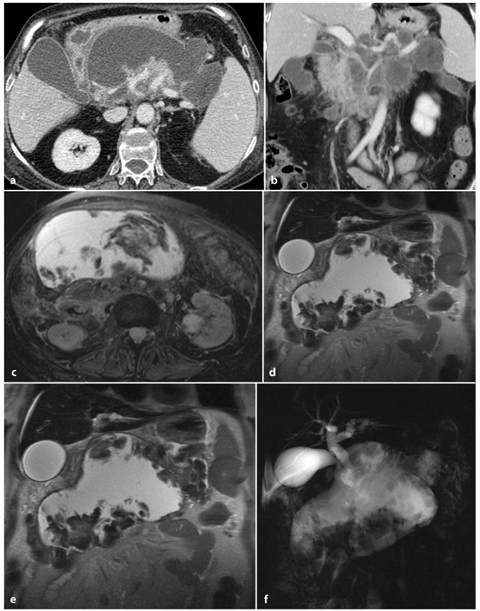

Fig. 6.4
Severe acute pancreatitis, with pancreatic necrosis involving more than 50% of the gland. a, b Axial and multiplanar reformatted paracoronal venous-phase computed tomography images show a large hypodense septated fluid collection replacing the pancreatic body and tail. Only the pancreatic head is preserved. c, d Axial and coronal T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) images show that the fluid collection has an inhomogenous hyperintense content with hypointense areas, attributed to necrotic debris and hemorrhagic clots. e An axial T1- weighted volumetric interpolated breath-hold examination (VIBE) image obtained in the arterial phase shows the large necrotic area replacing the pancreatic body and tail, and the pancreatic head with focal necrosis in its contest. f A magnetic resonance cholangiopancreatography image shows the huge necrotic area, with deletion of the main pancreatic duct
6.6 Acute Pancreatitis: Etiology
Acute pancreatitis can be caused by several etiological factors, which can be divided into: mechanical, metabolic, vascular and infectious. Gallstone disease (40% of cases) and chronic ethanol abuse (30% of cases) are the most common causes [25].
Other causes of acute pancreatitis are: hypertriglyceridemia; hypercalcemia; ERCP; pancreatic trauma; drugs (azathioprine, 6-mercaptopurine, sulfonamides, estrogens, tetracycline, anti-epileptics, anti-retrovirals and furosemide; posterior penetrating duodenal ulcer; ischemia and vasculitis; pancreatic neoplasms; infections (mumps, coxsackievirus, acariasis, herpesvirus and cytomegalovirus); hereditary conditions (i.e., mutations in the gene for the cationic trypsinogen enzyme: PRSS1); cystic fibrosis; anatomical abnormalities of the pancreaticobiliary tract (e.g., pancreas divisum); and autoimmunity.
Acute pancreatitis that does not have any apparent cause is known as “idiopathic” (20–30% of cases).
6.7 Biliary Pancreatitis
It is generally agreed that biliary pancreatitis is caused by either a transient or a persistent obstruction of the ampulla of Vater by biliary sludge or calculi.
Universal use of urgent ERCP has revealed that the incidence of common bile duct stones in patients with biliary pancreatitis is about 25%. For this reason, it is widely accepted that in every patient with acute pancreatitis the biliary tree must be accurately evaluated to assess for the presence of stones.
Because of the relative low sensitivity of CT for detecting biliary calculi, patients with biliary pancreatitis require additional imaging, usually US, to exclude the presence of biliary stones. However, because the sensitivity of MRCP is high, a single imaging evaluation is possible when patients are referred for MRI. The sensitivity and specificity of MRCP in detecting common bile duct stones are 81–99% and 85–99%, respectively, and superior to that of CT (45–85% sensitivity) and US (20–65% sensitivity) [31]. The sensitivity of MRCP for stone detection is similar to that of ERCP for the detection of common bile duct stones, but ERCP is associated with a higher failure rate of 3–11% [32], a complication rate of 7%, and a mortality rate of 1% [33].
It should also be noted that, although in many patients biliary sludge and/or debris are the cause of pancreatitis and these patients may benefit from a sphincterectomy to aid passage of the material, biliary sludge and many common biliary duct stones are usually asymptomatic.
As a result of this, only a minority of patients with gallstone pancreatitis may benefit from pre-operative ERCP as a therapeutic measure. Therefore, in the absence of biliary sepsis, the biliary tree should be imaged with a non-invasive method.
MRCP has a reported negative predictive value of 98% for detection of common bile duct stones in patients with acute gallstone pancreatitis; this helps to select those patients requiring stone extraction by ERCP [34] and to determine which patients would benefit from retrograde cannulation of the ducts [32].
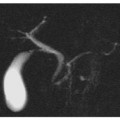
Moreover, MRCP and secretin-MRCP provide an accurate and non-invasive evaluation of the entire biliary system, facilitating the detection of some rare and complex pathologies, such as intrahepatic biliary stones, Mirizzi syndrome and choledochal cysts (Fig. 6.5). These techniques may also show a normal main pancreatic duct, which is a finding that could exclude acute pancreatitis in a setting of chronic pancreatitis, where a dilated or obstructed main pancreatic duct is commonly seen (Fig. 6.5) [14, 35].
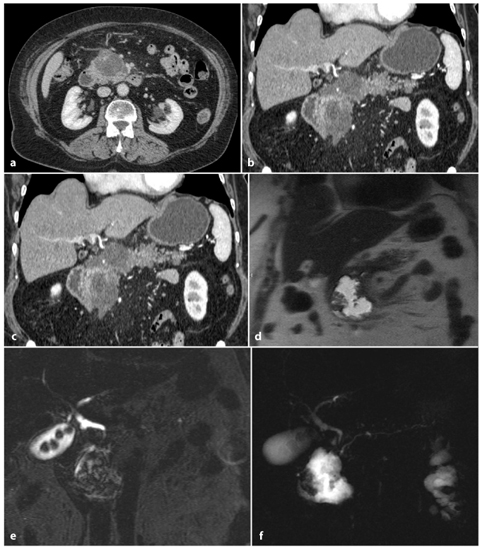

Fig. 6.5
Biliary acute pancreatitis. a, b Axial and multiplanar reformatted paracoronal contrast-enhanced computed tomography images taken during the portal venous phase show a hypodense fluid collection in the pancreatic head and neck. c, d Axial and coronal T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) images show an inhomogenous, hyperintense fluid collection with hypointense areas, attributable to necrotic debris. e A coronal T2- weighted fat-suppressed image shows gallstones and a slightly dilated common bile duct. f A magnetic resonance cholangiopancreatography image confirming the presence of gallstones, a dilated distal common bile duct and a dilated pancreatic duct, which is probably a result of fluid collection in the pancreatic head and neck
Furthermore, Romagnuolo et al. have reported that MRCP (or endoscopic US) is significantly more cost-effective than the selective use of ERCP for the identification of choledocholithiasis in patients with acute pancreatitis [36]. MRCP is also associated with a lower incidence of recurrent or ERCP-induced pancreatitis.
6.8 Pancreatic Duct Disruption
The management of pancreatic duct disruption is complex and depends on multiple factors, including the cause of the disruption, the clinical condition of the patient, parenchymal and ductal anatomy, and the degree of disruption.
The causes of pancreatic duct disruption include acute pancreatitis, chronic pancreatitis, surgery and trauma.
Small side-branch disruptions can heal without long-term sequelae, but disruption of the main duct can result in secondary recurrent acute pancreatitis, strictures, pancreatic atrophy, and eventually endocrine and exocrine insufficiency. Thus, timely diagnosis of pancreatic duct injury is paramount in reducing pancreas-specific morbidity and mortality.
Current treatment options include surgery, endoscopic intervention and conservative management [3].
Correct diagnosis of duct disruption, its location, and size of the leak are essential for choosing the appropriate treatment.
Historically, only ERCP has been able to provide dynamic information about continuing duct disruption. Despite this, ERCP can fail to show the disruption because of duct obstruction proximal to it, and the overfilling of a disrupted duct is actively discouraged because of the risk of sepsis and hemorrhage [37].
Although helical CT can depict pancreatic lacerations and pseudocysts, the continuity of the laceration and pseudocysts is more completely demonstrated with MRCP (Fig. 6.6). MRCP thus provides all the information that is available from CT, and information on the duct, which is often more complete than that obtained with ERCP. MRCP obtained with state-of-the-art technology is diagnostic in 95–99% of cases [38, 39]. Complete visualization of normal caliber pancreatic ducts in the pancreatic head and body has been reported in 97% of patients, and in the pancreatic tail in 83% of patients [39]. Dilated pancreatic ducts are essentially always visualized with MRCP. In contrast, ERCPs are diagnostic in 80–90% of cases because of the inability to cannulate the ducts in 10–20% of patients [40].
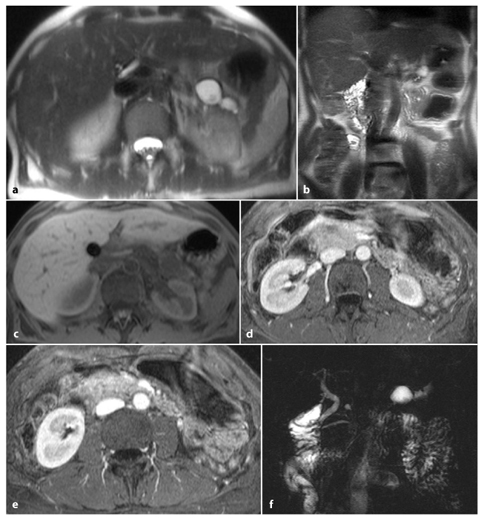

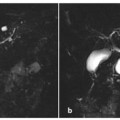
Fig. 6.6
Post-necrotic acute pancreatitis, with important main pancreatic duct disruption. Axial and coronal T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) (a, b) and axial fat-supressed gradient refocused echo (GRE) (c) images show enlargement of the pancreatic head and the presence of a small fluid collection corresponding to the pancreatic body/tail passage. d, e Axial T1-weighted volumetric interpolated breath-hold examination (VIBE) images obtained during the arterial phase show enlargement of the pancreatic head, with small hypointense areas in its content, and decreased pancreatic enhancement in the pancreatic body and tail. f A magnetic resonance cholangiopancreatography image shows pancreatic duct interruption in the pancreatic body, with slight dilatation of the main pancreatic duct in the tail and a pseudocyst at this level
To summarize, MRCP is a non-invasive technique that can depict the following: the entire pancreatic anatomy, both parenchymal and ductal; peri-pancreatic fluid collections; disruption, including a leak, upstream of an obstructed duct [41].
6.9 Intraductal Papillary Mucinous Neoplasms and Acute Pancreatitis
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a rare pancreatic tumor characterized by intraductal proliferation of mucin-producing cells, with hypersecretion of mucin, which leads to cystic dilatation of the involved ducts. The usual clinical presentation is recurrent episodes of pancreatitis as a result of mucin hypersecretion and temporary obstruction of the main pancreatic duct [42].
IPMNs are most frequently localized in the main duct of the head of the pancreas [43]. Clinical symptoms of IPMN are different from those of pancreatic adenocarcinoma, and about one-quarter of patients have pancreatitis-like symptoms (episodes of epigastric pain, hyperamylasemia), often for many years [44].
Symptoms of acute pancreatitis are caused by temporary complete obstruction of the main pancreatic duct by viscous mucin. About half of the patients develop pancreatic insufficiency with weight loss, diabetes and/or steatorrhea [45]: these symptoms of chronic pancreatitis are caused by permanent or prolonged occlusion of the main duct in the pancreatic head or by large amounts of viscous mucin that cannot be washed off by pancreatic secretions [46]. The main clinical problem is to differentiate IPMN from chronic pancreatitis with relapsing episodes. In fact, most undiagnosed IPMNs are wrongly interpreted as chronic pancreatitis.
Acute pancreatitis in IPMNs is not severe and often recurs without treatment. Moreover, in patients with an episode of pancreatitis, the finding of pancreatic cysts is often attributed to pseudocysts or fluid collections that make the diagnosis of IPMN less likely [47].
Communication between the duct and the abnormal cystic structure can be shown with MRI and MRCP (Fig. 6.7) [48].
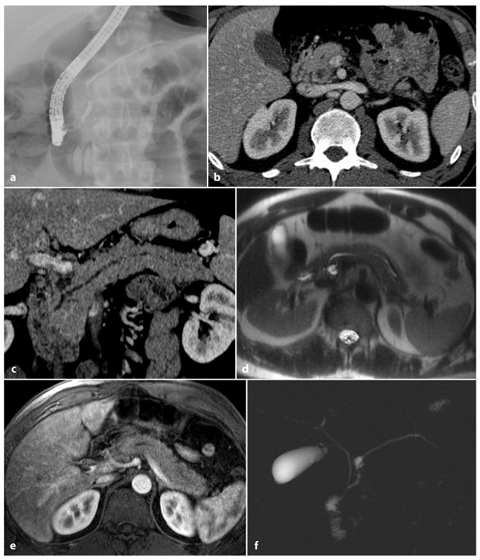

Fig. 6.7
Non-severe acute pancreatitis, with an intraductal papillary mucinous neoplasm. a An endoscopic retrograde cholangiopancreatography image shows a normal pancreatic duct. b, c Axial and multiplanar reformatted paracoronal computed tomography images show mild enlargement of the pancreas, with a slightly dilated pancreatic duct and a cystic lesion in the pancreatic head. d An axial T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) image showing slight enlargement of the pancreas, with inhomogenous pancreatic enhancement following gadolinium-chelate administration, and confirmation of the cystic lesion in pancreatic head. e An axial T1-weighted volumetric interpolated breath-hold examination (VIBE) image obtained in the arterial phase shows a decreased diffuse enhancement in the pancreatic parenchyma. f A magnetic resonance cholangiopancreatography image shows a small cystic lesion in communication with the pancreatic duct, confirming the diagnosis of intraductal papillary mucinous neoplasm
Although the classic diagnostic role of ERCP in the evaluation of IPMN has been challenged to some extent by the combined use of MRCP and endoscopic US, ERCP has a distinctive diagnostic role to play when a diagnosis is not clear on cross-sectional images [49].
6.10 Pancreas Divisum and Acute Pancreatitis
Pancreas divisum affects 5–10% of the population and is considered to be a congenital anomaly of pancreatic ductal configuration.
When the dorsal and ventral pancreatic ducts fail to fuse, pancreas divisum occurs; this means that most of the glandular parenchyma is drained by the dorsal duct through the minor papilla. Although most people with pancreas divisum have no clinical disease, it has been suggested that pancreas divisum may cause unexplained abdominal pain and recurrent episodes of acute pancreatitis or mild chronic pancreatitis because of the presence of a relative obstruction to the drainage of pancreatic secretions at the minor papilla. The inherently small diameter of the minor papilla causes increased pressure in the dorsal pancreatic duct [50–53].
For many years, ERCP was considered to be the only technique able to diagnose pancreas divisum, and recently Asayama et al. confirmed that ERCP is superior to multidetector row CT in assessing the presence of a ductal anomaly compatible with pancreas divisum [54].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree