7 Acute Scrotal Pain: Diagnosing with Color Duplex Sonography The differential diagnosis for acute scrotal pain includes testicular torsion (spermatic cord torsion), infection (epididymitis, orchitis, epididymo-orchitis), torsion of the appendix testis, trauma, incarcerated or strangulated inguinal hernia, hemorrhage into or necrosis of a testicular tumor, vasculitis, spermatic cord thrombosis or compression (during inguinal hernia surgery), and complications of vasectomy. The most common causes vary with age, but in all age groups, spermatic cord torsion and infection are the leading causes of acute scrotal pain. In adults, infection is more common than torsion, but in children (including neonates), torsion is more common. Torsion of the appendix testis or appendix epididymis is also relatively common in children. The remaining causes of acute scrotal pain are less common in all age groups (Table 7–1). Differentiating torsion from infection is an urgent problem. Torsion can lead to infarction of the testis within a few hours, so rapid reduction of the torsion is necessary. The mechanism of infarction is initially venous and lymphatic obstruction, later followed by arterial obstruction. The soft lymphatic and venous structures are prone to obstruction at lower degrees of torsion than are the thicker-walled testicular arteries. The rapidity with which torsion causes infarction varies with the degree of torsion. Infarction may not occur for days if there is only 90 degrees of torsion. On the other hand, 720 degrees of torsion may cause infarction within 2 hours. Intermediate degrees of torsion can lead to infarction in intermediate lengths of time. An underlying anatomical abnormality, the “bell-clapper deformity,” predisposes the patient to torsion in most cases. In the bellclapper deformity, the normal, “low, broad attachment” of the tunica vaginalis to the posterior surface of the testis is absent. Instead, the tunica vaginalis attaches in an abnormally high position to the spermatic cord, and the mesorchium is abnormally long. The lack of attachment of the testis to the tunica vaginalis predisposes the testis to torsion around the high point of attachment to the spermatic cord. Thus, testicular torsion should more properly be termed spermatic cord torsion. The bell-clapper deformity is usually bilateral, predisposing both sides to torsion. The urgent reduction of torsion can be performed surgically or nonsurgically under local anesthetic. Regardless of whether reduction of torsion is surgical or nonsurgical, orchiopexy is required to prevent repeat torsion at a later date. Orchiopexy is almost always performed bilaterally because the bell-clapper deformity, which is the underlying predisposition to torsion, is usually present bilaterally. The patient’s clinical history can be helpful, but is sometimes quite atypical. The precipitating factor is thought to be an unusually strong contraction of the cremasteric muscle, which can be caused by trauma, strenuous exercise, or sexual activity. However, in many patients the onset of pain occurs during sleep, without any apparent cause for forceful contraction of the cremasteric muscle. The onset of pain in spermatic cord torsion is usually acute. Gradual onset of pain is more typical of infection, but up to 25% of torsion patients also have gradual onset of pain. Spontaneous detorsion does occur, and careful questioning sometimes reveals a past history of repeated episodes of scrotal pain due to intermittent spontaneously resolving episodes of torsion. Urinary symptoms such as dysuria are relatively rare in torsion in comparison with epididymitis.
Differential Diagnosis
Diagnostic Evaluation
Differentiations Torsion from Infection
Children Torsion Infection Appendage torsion Tumor Trauma Hernia Spermatic versus occlusion Vasculitis Adults Infection Torsion Appendage torsion Tumor Trauma Hernia Spermatic versus occlusion Vasculitis |
In patients in whom the history and physical examination are not classical for torsion, ultrasound with Doppler can be used to help differentiate torsion from infection. Our ability to demonstrate specific gray-scale patterns of edema within the testes, epididymis, and spermatic cord has improved. The sensitivity of color duplex sonography and power Doppler sonography for demonstrating the relatively slow and sparse flow within the testes has also improved markedly over the years. The combination of improved gray-scale imaging and Doppler capabilities now make ultrasound and Doppler the imaging procedures of choice for evaluation of acute scrotal pain.
Ultrasound Imaging
Ultrasound Equipment and Machine Setup
Scrotal sonography requires both high-frequency, high-resolution, near-field, real-time, gray-scale imaging and superb color and/or power Doppler sensitivity. These are best accomplished with high-frequency electronically focused transducers in the 10 to 12 MHz range. In children, frequencies as high as 17 MHz can be used. Lower frequency transducers, such as 5 MHz linear array transducers, are designed for peripheral vascular Doppler, not small parts imaging, and will less frequently show normal intratesticular flow than will higher-frequency transducers. Failure to show normal flow on the contralateral asymptomatic side reduces the value of Doppler in assessment of acute scrotal pain caused by torsion. In addition to Doppler sensitivity limitations, most 5 MHz transducers also have short-axis fixed focal lengths that are too deep for optimal scanning of the scrotal contents, especially in children. However, in patients with large hydroceles or hematoceles, severe testicular enlargement, a large testicular mass, or severe skin swelling, the greater focal length of the 5 MHz linear probe may be an advantage.
Power Doppler imaging is more sensitive than color Doppler flow imaging for showing relatively slow flow in small vessels, such as those within the substance of the normal testes. This is especially true in young boys and very old men, who tend to have less flow within the testes. Power Doppler is also less angle dependent than color Doppler, showing small vessels that course near 90 degrees to the Doppler beam better than does color Doppler. Being able to demonstrate flow within the normal contralateral asymptomatic testis is critical to the diagnosis of torsion because failure to do so makes lack of demonstrable flow in the symptomatic testis meaningless, and in a few cases power Doppler can show normal flow better than color Doppler does.
Some ultrasound equipment enables the user to down-shift the Doppler frequency to a lower frequency than is normally used for imaging (i.e., the Doppler frequency on a 10 MHz linear transducer might be 7 MHz rather than 10 MHz). This downshift allows better penetration for peripheral vascular applications and also allows a greater degree of beam steering to optimize angles of incidence in peripheral vessels, such as the carotid and femoral arteries. However, downshifted Doppler frequencies and beam steering both reduce Doppler sensitivity and generally should not be used for evaluating intratesticular blood flow except in rare cases where large masses or marked scrotal enlargement makes penetration with higher frequencies suboptimal. If the vessels of interest within the scrotum are coursing at an obtuse angle of incidence, it is easy to move the probe along the surface of the scrotum to a position in which the angle of incidence is more acute. Angles as close to 0 degrees as possible should be sought and are usually possible. Sliding the transducer parallel to its long axis to a different location on the surface of the scrotum is a better way to improve angles of incidence than is beam steering.
Doppler sensitivity settings should be maximized. This is especially important in young children and old men, who have less flow and slower flow than young adult and middle-aged men. Full color and pulsed Doppler power, low-velocity scales, low wall filters, and high gray-scale-write-priorities maximize sensitivity. Many units, due to U.S. Food and Drug Administration requirements, boot up at low-power settings for safety purposes. These low-power settings can adversely affect Doppler sensitivity for the detection of normal intratesticular blood flow, especially in children and elderly men. The risk:benefit ratio of Doppler imaging in these patients demands full power, if flow within the asymptomatic testis cannot be demonstrated at the default low-power setting. Low-velocity scales (pulse-repetition frequency) and low wall-filter settings also maximize our chances of demonstrating flow. The ultrasound unit can usually only write either color or gray scale to each pixel, but not both. The write-priority tells the unit how white a gray-scale pixel can be overwritten with color. Peripheral vascular applications, with large lucent vessels, require low write-priority settings (that is, toward the black end of the gray scale). In such cases, high write-priorities (toward the white end of the gray-scale spectrum) would result in too much artifactual color being written into echogenic tissues outside of the vessels. In contrast to large peripheral vessels, the testes and epididymis are relatively echogenic. Many of the vessels within them that are being interrogated are too small to resolve on the B-mode image. Therefore, color flow must be displayed on a relatively white background by using a high write-priority. If the B-write-priority settings are too low (like those used for peripheral vascular imaging), color may not be written onto the image of the testis, even when the unit has correctly detected it. Some ultrasound units do not have a separate control for write-priority and have combined control of this parameter with other parameters (i.e., threshold control).
In most cases, color Doppler or power Doppler imaging alone is sufficient to distinguish between infection and torsion by showing markedly increased or decreased flow within either or both the symptomatic testis and epididymis. However, occasionally, obvious color or power Doppler asymmetry may not be demonstrable. In such cases, pulsed Doppler spectral analysis can be helpful. As is the case for color and power Doppler imaging, electronic beam steering reduces the sensitivity of pulsed Doppler spectral analysis and should not be used. In our experience, it is possible to maneuver the transducer on the surface of the scrotal skin to create an angle of incidence of the pulsed Doppler beam that is near 0 degrees without steering in virtually all cases. We have also found that using a wider sample volume improves the quality of the spectral tracing when the vessel being interrogated is small and the angle of incidence is near 0 degrees.
Occasionally, the testis is too long to measure with a standard 38 mm long 10 or 12 MHz linear array transducer. Five centimeter long transducers, trapezoidal beam shapes, extended field of view, or 5 MHz curved linear array transducers all offer workarounds to this problem.
Sonographic and Duplex Technique
Ultrasound examination of the scrotum in patients with acute scrotal pain should always include Doppler studies in addition to gray-scale imaging. On the other hand, Doppler evaluation is usually not necessary when the patient has a nonpainful, nontender, palpable nodule or mass. In patients in whom the clinical index of suspicion of torsion is high and in those who have gross enlargement of the testis, color Doppler flow imaging should be used early in the examination to make as prompt a diagnosis of torsion as possible. A color or power Doppler examination of the symptomatic testis can usually be performed very quickly. If there is no demonstrable flow within the symptomatic testis, one should quickly make sure that flow is demonstrable in the contralateral asymptomatic testis. This will be discussed in greater detail later.
After the quick color or power Doppler survey, the entire symptomatic testis and epididymis should be scanned in real time gray scale to get an overall picture of the scrotal contents and alignment of the testis and epididymis. The contralateral asymptomatic side should also be quickly surveyed to get an idea about symmetry in size and echogenicity between the two sides and also to assess the position and alignment of the contralateral testis and epididymis. The spermatic cord should be inspected from several centimeters superior to the epididymal head to the tail of the epididymis. The presence of a complex hydro-cele and debris or adhesions within it should be noted. Intratesticular nodules or masses should be excluded, and the testes measured. The presence of a solid mass or nodule within the testis suggests the presence of a neoplasm. A complex cystic lesion within the testis may represent an abscess resulting from orchitis or necrosis resulting from infarction or tumor.
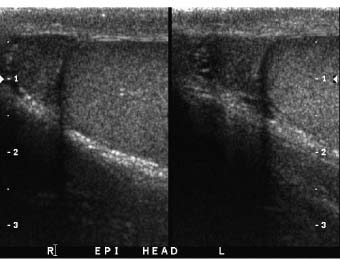
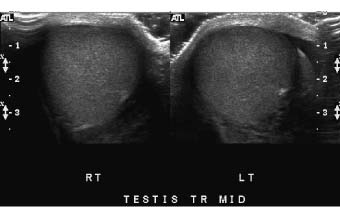
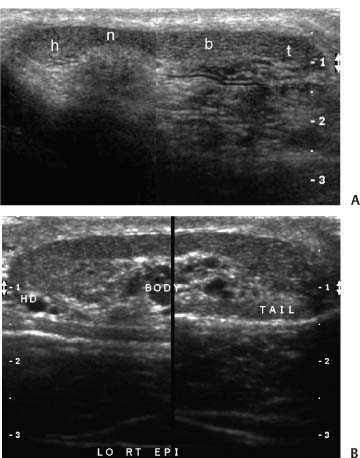
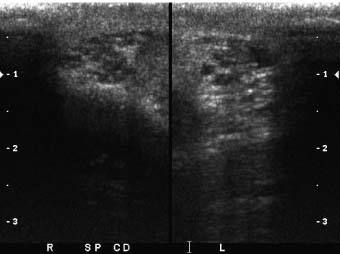
One advantage of scrotal–testicular color duplex sonography is that there is almost always a mirror-image contralateral normal structure with which the painful side can be compared. This advantage should be utilized to its fullest extent. When the color Doppler findings do not indicate torsion and the need for immediate surgery, we make this comparison by obtaining split-screen images of corresponding right and left intrascrotal contents in both transverse and longitudinal planes. Split-screen long-axis images of the epididymal heads (Fig. 7–1) and long- and short-axis split-screen images of both testes are recorded (Fig. 7–2). The epididymal tails are usually best shown on coronal views that are angled from anterosuperior to posteroinferior. Obtaining such a view often requires the patient to temporarily assume a frogleg position. The shape of the epididymis varies from straight (Fig. 7–3A) to C-shaped (Fig. 7–3B). Imaging the epididymal tails is very important because infection is often most apparent and manifests itself most definitively there. Transverse split-screen images of the spermatic cords just superior to the epididymal heads are also obtained (Fig. 7–4). Occasionally, scanning the spermatic cords within the inguinal canals can be helpful. We also obtain an oblique short-axis view through the median raphe of the scrotum to compare the size and echogenicity of the testes on a single image. It is important that the angle of incidence of the beam into the testes be bilaterally symmetrical on this view to minimize the chance that critical angle shadowing will make a testis artifactually look hypoechoic. Usually the left end of the probe must be angled inferiorly because the left testis usually lies lower than the right, but this relationship may be altered in torsion. It is important to make sure that all of the split-screen images are obtained symmetrically through the widest part of the structures being imaged and that the angles of incidence be as close to 90 degrees as possible to minimize artifactual shadowing and errant assessment of relative echogenicities and thicknesses (Fig. 7–5). The right and left testes, epididymi, and spermatic cords should be symmetrical in size and echogenicity.
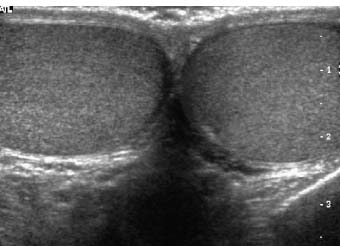
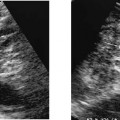
Figure 7–1 Long-axis split-screen images of bilaterally normal epididymal heads. The heads are triangular and symmetrical in size and shape. The transducer position needed to obtain these views varies not only from one patient to another, but from right side to left.
Figure 7–2 Split-screen transverse images through the normal right and left testes—size and echogenicity are symmetrical from right to left.
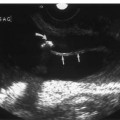
Figure 7–3 (A) Coronal or C view of normal straight epididymis. Combined view of entire length of normal epididymis shows the head (h), neck (n), body (b), and tail (t). (B) Coronal or C view of normal curved epididymis.
Demonstrating symmetrical sections through right and left sides may require grossly different probe orientations on the right and left sides because the position of the testes within the scrotal sac in normal individuals is often asymmetrical. In torsion, this asymmetry of position is even more pronounced. The relative position of the right and left sides is ascertained at the time of the initial survey.
Figure 7–4 Short-axis split-screen images of spermatic cords. These short-axis views were obtained through the spermatic cords just superior to the epididymal heads. The cords are symmetrical in size and echogenicity. Because of tortuosity of the cord, obtaining such views in nonredundant segments of cord can be difficult.
Figure 7–5 Oblique median raphe view through the medial half of each testis. This median raphe view shows the testes to be equal in size and echogenicity. Because the left testis is usually lower than the right, this view cannot be obtained in a true transverse plane and usually requires an oblique view with the left side lower.
We are not in favor of using a rolled-up towel to support the testis. Although this may offer symptomatic relief to a patient with epididymitis, it blocks inferior and coronal approaches to the testis and also pushes the scrotal contents superiorly, interfering with the evaluation of the epididymal heads, appendices, and spermatic cords above the testes. Additionally, the rolled-up towel redistributes physiological amounts of fluid within the scrotal sac posteriorly, where it cannot be used to outline small structures such as the appendix testis. Scanning without a rolled-up towel allows the scrotal contents to fall inferiorly and posteriorly and redistributes physiological hydroceles to the upper pole, nicely outlining appendices, epididymal heads, and spermatic cords. It also stretches out the epididymis, facilitating demonstration of the entire epididymis on a single coronal view.
The Doppler examination begins with color or power Doppler interrogation of the testes. We usually quickly evaluate the asymptomatic testis first to make sure that flow is detectable on the side on which torsion is not suspected. If no flow exists in the asymptomatic side, decreased or absent flow in the symptomatic side will have no meaning. If flow cannot be detected within the asymptomatic contralateral testis, velocity scale, wall filter, B-write priority, and Doppler power can be adjusted as needed to demonstrate flow. Increased flow in the symptomatic testis is always important, regardless of the contralateral findings. It indicates inflammatory hyperemia, which is almost always due to infection; in rare cases, it can be a manifestation of reactive hyperemia after spontaneous detorsion.
If flow is present in the asymptomatic testis, but absent or decreased in the symptomatic testis, then torsion is likely. Duplex sonographic interrogation with spectral tracings and complete color Doppler and duplex sono-graphic studies of the remainder of the intrascrotal contents are usually not performed because it is important for the patient to undergo surgery as soon as possible. Additional examinations in such patients may waste valuable time in which the patient could be prepared for surgery. It should be kept in mind, however, that severe infection, trauma, and inguinal hernia surgery can lead to ischemia of a testis, which appears similar to torsion on color Doppler flow imaging or power Doppler imaging. The pattern of oligemia can be helpful. Spermatic cord torsion tends to cause global absence or decrease in flow within the testis, whereas in ischemia caused by conditions other than torsion, the ischemic changes often appear focal or patchy rather than diffuse.
If flow is present in both testes but increased in the symptomatic testis, or if there appears to be normal and symmetrical flow in both testes, torsion is unlikely. Complete gray-scale imaging with split-screen images and color duplex sonographic examination of intrascrotal contents can then be performed. In some cases, spectral Doppler analysis will show asymmetries in velocities and resistivity indexes, even when color Doppler appears symmetrical. The goal of complete gray-scale and Doppler in assessing testes, epididymi, and spermatic cords is to detect a pattern of hyperemia that suggests a specific diagnosis. For example, abnormally high velocities and low resistivity indexes in the epididymis, but normal velocities in the testes, strongly favor a diagnosis of epididymitis over other causes.
It is very important to assess the intratesticular vessels (centripetal) as well as the more easily seen capsular arteries. In cases of missed torsion, the tunica vaginalis, the lining of the scrotal sac, can be markedly hyperemic. Because the inflamed tunica vaginalis may become adherent to the immediately adjacent tunica albuginea, reactive hyper-emia within the tunica vaginalis can be mistaken for flow within a capsular testicular artery, resulting in a missed diagnosis of torsion. By assessing intratesticular arteries rather than capsular arteries, this potential mistake can usually be avoided. As mentioned earlier, it is possible to create an optimal angle of incidence of near 0 degrees without electronically steering the ultrasound beam to maximize Doppler sensitivity.
In most cases the combined pattern of swelling, altered echogenicity, and increased or decreased flow on Doppler ultrasound examination suggest the exact cause of scrotal pain.
Normal Imaging and Doppler Findings
Normal testes, epididymi, and spermatic cords are bilaterally symmetrical in size and texture. The epididymi and testes are normally relatively homogeneous and have mid-level echogenicity. The spermatic cords have heterogeneous texture due to the venous plexus and abundant loose connective tissues they contain. The tortuosity of the spermatic cord makes it difficult to obtain precisely symmetrical images and to precisely measure the spermatic cord, but an eyeball estimate of size and echogenicity is usually possible. Marked asymmetries in size, shape, homogeneity, and echogenicity between right and left sides are abnormal.
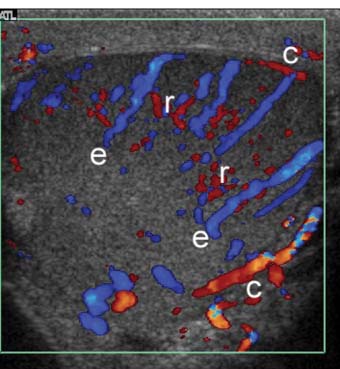
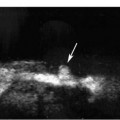
Figure 7–6 Color Doppler image through equator of testis. Capsular arteries (c) course on the outer surface of the testis, centripetal arteries (e) penetrate into the substance of the testis from peripheral to central, and recurrent rami (r) double back toward the surface of the testis. Demonstrating flow in centripetal arteries is important in excluding torsion because vessels within adherent tunica vaginalis can simulate capsular arteries in patients with “missed” torsion.
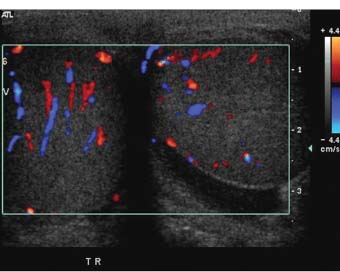
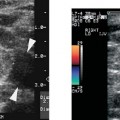
With current equipment, flow is demonstrable within the capsular arteries, the intratesticular vessels (centripetal and, sometimes, recurrent rami), in almost all adults within the reproductive age groups (Fig. 7–6). Demonstrable color Doppler flow should be roughly symmetrical in the right and left testes, with only minimal asymmetry in the number of vessels in right and left testes shown on oblique transverse median raphe views (Fig. 7–7). However, normal intratesticular flow can be more difficult to demonstrate in children, particularly very young children and newborns, and in very elderly men. Additionally, the intratesticular vessels pass through the testis in a few discrete vascular planes. If the ultrasound probe is parallel to the vascular plane on one side, but out of the vascular plane in the contralateral testis, the flow may appear falsely asymmetrical. Every effort should be made to compare comparable tissue planes within the two testes. The vascular planes of the testis are oriented in the long axis of the testis. Therefore, errors in assessment of vascularity are more likely to occur in the longitudinal planes than in transverse plans. The capsular arteries are usually best seen in segments that course at nearly a 0 degree angle of incidence to the beam. This most commonly occurs on the inferomedial surface of the testes. Multiple intratesticular vessels are visible in most adult testes, but may be more or less evident, depending upon the angle of incidence and scan plane.
Figure 7–7 Oblique median raphe color Doppler view can be helpful in comparing blood flow in the right and left testes on a single view. In normal individuals, flow should be roughly symmetrical on right and left sides.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree