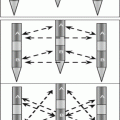
Fig. 10.1
Diagram of potential mechanisms of direct effect of IFN to antitumor growth
In a retrospective study, Someya et al. [15] investigated the role of IFN in HCC patients with HBV-related cirrhosis after curative operation; the results showed that IFN prevented tumor recurrence, especially in those with higher aspartate transaminase levels. Furthermore, the randomized controlled trials (RCTs) have been conducted to explore the effects of IFN for preventing recurrences in HCC patients after treatment. Lo et al. [16] conducted an RCT in a series of 40 patients with HBV-related HCC after hepatic resection. Patients treated with IFN-α2b, 10 MU/m2, three times a week for 12 weeks were compared with controls. The survival rates in the IFN-treated patients at 1st and 5th years after surgery were 97 % and 79 %, respectively, compared to 85 % and 61 % in the control. Multivariate analysis showed that IFN might decrease the risk of patient death. In a subgroup analysis, the 5-year survival rate in stage I/II patients did not show the difference between the IFN group and controls. However, the early recurrence rate in stage III/IVA HCC patients with IFN administered was significantly decreased, and the 5-year survival rate was improved from 24 to 68 %. Sun et al. [17] also demonstrated that postoperative IFN-α treatment postponed tumor recurrence and improved overall survival in HBV-related HCC patients after curative resection in an RCT study. Therefore, the therapeutic approach with IFN to prevent HCC recurrence after operation has been gradually adopted in clinical practice. Moreover, conventional IFNs have been replaced gradually by a new pegylated (PEG)-IFN as a common agent due to its advantages of higher response rate and simpler management method, which needs to be taken once a week only, rather than daily or three times a week.
Nevertheless, of the case–control studies reported previously, five cases were associated to hepatitis C [18–20] and two cases were associated to hepatitis B-related HCC [15, 21]. Suou et al. [18] assessed whether IFN-α could inhibit intrahepatic recurrence in small HCC patients with underlying disease of chronic hepatitis C after curative operation. The data from 40 patients who were with solitary, small HCC (lesion ≤3 cm in diameter), underlying chronic hepatitis C, and age ≤70 years were analyzed. Among them, 18 patients were treated with IFN-α for 6 months after HCC operation and 22 patients without IFN-α therapy were used as controls. In which, six patients (33 %) in the IFN group showed sustained response, but the incidence of local recurrence did not show the significant difference (6 % vs. 9 %, P >0.05). The cumulative incidences of distant recurrence in controls vs. the IFN group were 9 % and 6 % at year 1, 27 % and 11 % at year 2, 63 % and 18 % at year 3, 76 % and 28 % at year 4, and 82 % and 28 % at year 5, respectively, with a significant difference between the two groups. Six patients (27 %) in the non-IFN-treated group were dead with the progress of HCC, but all patients in the IFN-treated group were alive (P <0.05). The pilot study demonstrated that IFN-α therapy for small HCC patients after curative operation could inhibit intrahepatic recurrence in the residual liver and improved prognosis of hepatitis C virus-related HCC. Hung et al. [20] reported that antiviral therapy suppressed tumor recurrence and influenced overall survival rate in patients with HCV-related HCC, who had complete ablation of nodules by nonsurgical treatments. Twenty HCC patients with three or less HCV-related nodules were treated with percutaneous tumor ablation and/or transcatheter arterial embolization combined with interferon (IFN; 3 or 5 million units of IFN alpha-2b thrice a week) plus ribavirin (1000–1200 mg per day) for 24–48 weeks after complete ablation of lesions. During the same period, additional 40 age- and sex-matched patients with similar characteristics of tumors (sizes, numbers, and treatment modalities) and severity of liver disease were recruited as controls. In 20 patients with antiviral therapy, 16 completed therapy and 10 showed a sustained response with normalization of alanine aminotransferase and negative HCV-RNA at 6 months after therapy completion. Since treated child B patients experienced severe side effects (most patients then discontinued antiviral therapy), clinical outcomes were analyzed only in the treated child A patients (n = 16) vs. controls (n = 33). There was no significant difference in the local recurrence incidence in patients with sustained response compared to those without response or subjects as controls. However, the second recurrence-free interval and survival in patients with sustained response was significantly longer than those in patients without response and subjects in controls. These results indicated that successful antiviral therapy for HCV-related HCC patients after nonsurgical tumor ablation may lower tumor recurrence rate and prolong the survival. Qu et al. [21] retrospectively analyzed the effect of IFN-α therapy on survival and tumor recurrence in patients with HBV-related HCC after curative resection. A total of 568 HBV-related HCC patients had undergone curative resection. Among them, 101 patients received postoperative IFN-α therapy (5 million units, 3 times a week for 18 months) with median follow-up of 53.3 months. The results indicated that patients with postoperative IFN-α therapy showed higher overall survival rates, whereas no significant difference was found in disease-free survival rates between the two groups. Multivariate analysis revealed that postoperative IFN-α therapy was an independent factor for overall survival and could significantly reduce the risk of early tumor recurrence. Therefore, IFN-α given after curative resection could inhibit the early tumor reappearance and improve the overall survival in patients with HBV-related HCC.
The tumor recurrence and survival rate were evaluated similarly in all of these RCT studies. Significant differences were shown of the survival rate in subjects with a prolonged IFN administration. Improvements of survival rate were observed in one study with 6-month administration [18, 20] and another study with a median administration of 4.7 years [22]. A significant difference of tumor recurrence rate (including the rates of the second and third recurrences) was noted in all three studies. It was also reported recently that the tumor recurrence was significantly suppressed, and the survival was prolonged in patients administered with IFN for 2 years or longer [23]. Also, the recurrence rate of HBV-related HCC and the hazard ratio were lowered in IFN-treated patients than those in patients without IFN treatment [15]. IFN administration has also been reported to increase the overall survival rate [21]. These results suggested that the effect of IFN for preventing the tumor recurrence might be through the mechanisms of suppressing the inflammation or direct anticancer effect to both hepatitis C and B patients.
In addition, IFN has been proposed [19, 22] to be used at a low dosage for longer term consecutively as a maintenance therapy to improve the ALT level and expect its direct anticancer effect, rather than to eliminate the virus. In a report about IFN maintenance therapy, a total of 127 HCC patients (tumor diameter ≤3 cm; number of tumors ≤3) were performed RFA curatively. Among them, 43 patients received 3,000,000 IU IFN-α2b twice per week or PEG IFN-α2a 90 μg once per week or once per 2 weeks without discontinuation (IFN group maintenance). Meanwhile, additional 84 patients did not receive IFN treatment (control group). The data showed that the 5-year survival rate was 66 % in the control group and 83 % in the IFN group maintenance (p = 0.004). Multivariate analysis using the Cox proportional hazards model identified IFN maintenance therapy as an independent risk factor for survival, and the risk ratio was 0.21 (95 % CI: 0.05–0.73). Therefore, the authors concluded that the curative protocol of IFN at low dose for long-term maintenance after RFA operation significantly delayed the first recurrence, prevent the second and third recurrences, and consequently improved survival (Table 10.1) [22, 24].
Table 10.1
Therapeutic effect of interferon to patients with hepatocellular carcinoma (HCC) after surgical procedure
Study | With vs. without INF (n) | Etiology | Follow-up | HCC treatment modality | P value (recurrence) | P value (survival) |
|---|---|---|---|---|---|---|
Someya et al. | 11 vs. 69 | HBV | 3 years | Ablation | NS (P = 0.0139) | NA |
Lo et al. | 40 vs. 40 | HBV/HCV | 5 years | Resection | P < 0.05 (early recurrence) | P = 0.038 (stage III/IVA) |
Sun et al. | 118 vs. 118 | HBV | 36.6 months (median) | Resection | P < 0.048 | P < 0.0003 |
Suou et al. | 18 vs. 22 | HCV | 5 years | Resection/RFA | P < 0.01 | P < 0.05 |
Sakaguchi et al. | 24 vs. 33 | HCV | 3 years | RFA | P < 0.02 | NS |
Hung et al. | 20 vs. 40 | HCV | 6 months | RFA/TACE | P = 0.0141 | NS |
Qu et al. | 101 vs. 467 | HBV | 53.3 months (median) | Resection | P = 0.005 (early recurrence) | P = 0.01 |
Kudo et al. | 43 vs. 84 | HCV | 5 years | RFA | P < 0.05 | 0.004 |
Ikeda et al. | 77 vs. 302 | HCV | 5 years | RFA | P < 0.05 | NA |
According to Jeong et al. [25], the Child–Pugh score did not show the difference in IFN-treated patients, but was significantly deteriorated in controls during the follow-up period. The percentage of patients who were unable to be a candidate for surgery due to extensive cancerometastasis or worsening liver function was increased in the control group than those in the IFN group. In summary, the regimen of IFN therapy with long-term maintenance at low dosage seemed able to significantly prevent or delay the recurrence of HCC and the occurring of secondary carcinogenesis by preserving the liver function in an adequate state or by its direct anticancer effect, which made it possible to detect a slight recurrence and improve the survival rate while disease was still staying in a relatively early stage and the curative operation was allowed again. In addition, the performance of noncurative treatments such as transarterial chemoembolization which would damage the liver function and IFN therapy which might preserve the liver function was required to be considered meticulously for improving the survival rate [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree