CHAPTER 17 Adrenal Glands
CLINICAL CONSIDERATIONS
Anatomy of the Adrenal Glands
Histologic Anatomy
Histologically, each adrenal gland is composed of two different types of tissue with different functions: the peripheral cortex and the central medulla. The adrenal cortex is derived embryologically from coelomic mesoderm that develops into three stratified zones of cortical tissue (zona glomerulosa, zona fasciculata, and zona reticularis) that are vital to fluid-electrolyte homeostasis. The adrenal medulla is derived from neural crest ectoderm and produces a variety of catecholamines that regulate hemodynamics and respond to stress. Table 17-1 provides a summary of the regional production of hormones.
Table 17-1 Adrenal Endocrine Mediators by Location
| Location | Product |
|---|---|
| Adrenal Cortex | |
| Zona glomerulosa | Aldosterone |
| Other zones | |
| Adrenal Medulla | |
Cross-Sectional Anatomy
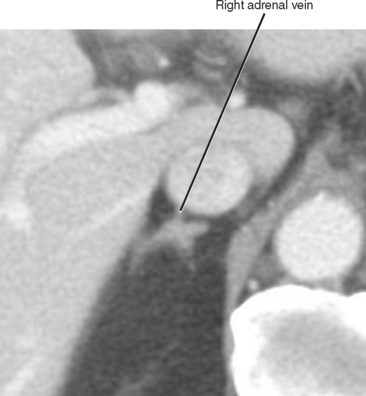
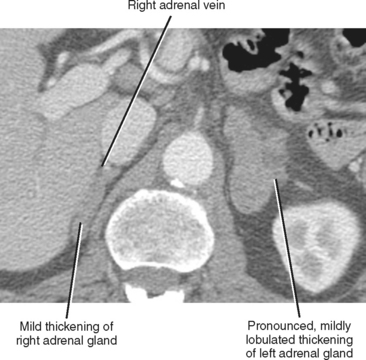
The normal adrenal glands reside in a superior and anteromedial recess of the perirenal space, and adhere to the innermost layer of the perirenal fascia at this location. The right adrenal gland can usually be identified in a location superior to the right kidney with some tissue protruding between the posterior margin of the inferior vena cava and the medial portion of the right diaphragmatic crus. Often, the entire right adrenal gland is superior to the kidney, although a small portion may extend inferiorly anterior to the kidney. The short, right adrenal vein can be identified in most cases (Fig. 17-1). The adrenal vein is occasionally a useful landmark for identifying the adrenal gland when a mass distorts structures in the suprarenal region.
Laboratory Findings Relevant to the Adrenal Gland
Laboratory Analysis of Adrenal Cortical Function
Cortisol production in the adrenal cortex is regulated through a feedback mechanism to prevent overproduction. A dexamethasone suppression test introduces exogenous steroid that should suppress the normal adrenal cortex. Failure of suppression indicates unregulated cortisol production (Cushing syndrome).
COMPARISON OF IMAGING MODALITIES
Table 17-2 presents a comparison of various imaging modalities for assessment of the adrenal glands.
Table 17-2 Imaging the Adrenal Glands: Relative Roles of Various Modalities
| Modality | Role |
|---|---|
| Ultrasound | |
| CT | |
| MRI | |
| PET-CT | 18F-FDG is taken up in greater quantity by cells with more rapid metabolism (including cancer cells) |
| MIBG |
CT, Computed tomography; 18F-FDG, F-18 fluorodeoxyglucose; MIBG, metaiodobenzylguanidine I123; MRI, magnetic resonance imaging; NCCT, noncontrast-enhanced computed tomography; PET, positron emission tomography.
CHARACTERIZING ADRENAL MASSES
First Things First: Is It Really the Adrenal Gland?
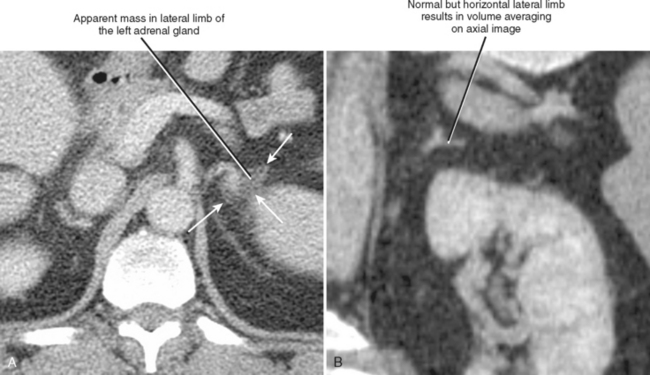
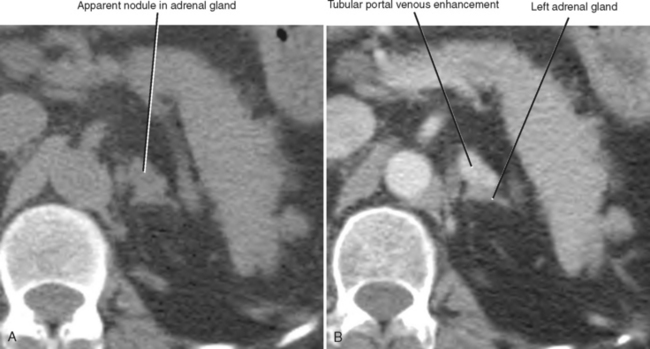
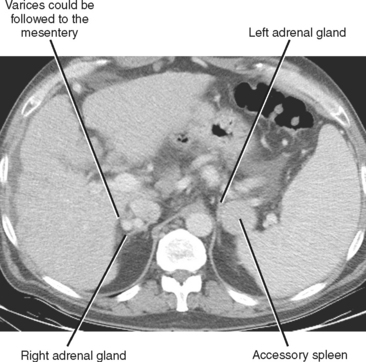
A number of normal and abnormal structures can mimic an adrenal mass. Adrenal mimics are much more common on the left side because of potential collateral venous pathways around the spleen and proximity of the left adrenal gland to the stomach and pancreas. Having a high index of suspicion is helpful in identifying subtle signs, such as traces of air in a gastric diverticulum, enhancement similar to nearby splenic tissue, or contiguity with adjacent vascular structures. Table 17-3 lists the most common mimics of adrenal masses.
Table 17-3 Mimics of Adrenal Mass
| Mimics | Distinguishing Features |
|---|---|
| Plane-dependent pseudolesion on computed tomography (Fig. 17-5) | |
| Splenic varices (Fig. 17-6) | |
| Tortuous splenic artery | |
| Gastric diverticulum | |
| Accessory spleen (Fig. 17-7) | • Uptake on technetium-99m-labeled, heat-damaged, red blood cell study or liver-spleen scan with single-photon emission computed tomography |
| Pancreatic pseudocyst | |
| Tumors of the celiac ganglion (ganglioneuroma) | |
| Tumor of adjacent organ (e.g., kidney, stomach) or perirenal space (e.g., liposarcoma) |
Clinical Context
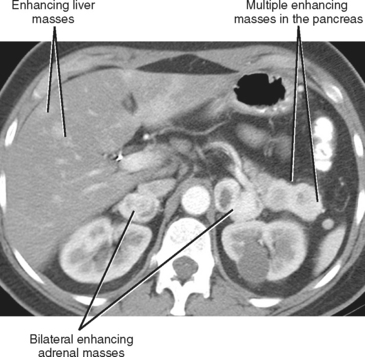
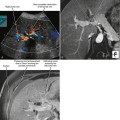
A known history of cancer increases the likelihood that a given adrenal mass is metastatic disease. More specific patterns of multiorgan involvement suggest syndrome-associated adrenal neoplasms, such as pheochromocytoma associated with neurofibromatosis, multiple endocrine neoplasia or von Hippel–Lindau syndrome (Fig. 17-8). A retroperitoneal mass in a young girl with hypertension and associated masses in the lung (pulmonary chondroma) or stomach (gastrointestinal stromal tumor) is suspicious for an extraadrenal paraganglioma in Carney’s triad.
Mass Size, Shape, and Number
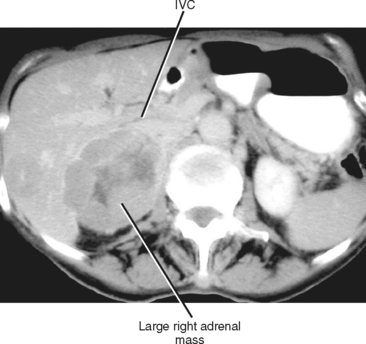
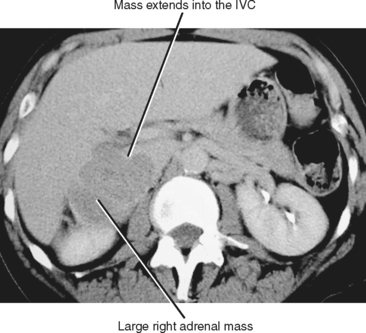
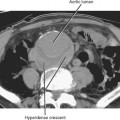
The size of an adrenal mass is a relatively strong discriminator between benign and malignant lesions. Most adrenal adenomas are small, averaging just larger than 2 cm in diameter in most series. At autopsy, only 2% of adrenal adenomas are larger than 4 cm and 0.03% are larger than 6 cm. Metastases, adrenal cortical carcinomas, and pheochromocytomas are often large at time of presentation (Fig. 17-9 and 17-10). Ninety-two percent of adrenal cortical carcinomas are larger than 6 cm at time of diagnosis. Thus, a small adrenal mass (<3 cm) with no suspicious imaging features in a patient without known risk for metastases can be followed with serial imaging unless an additional study (noncontrast computed tomography (CT), washout CT, or magnetic resonance imaging [MRI]) can characterize the mass definitively. A mass larger than 5 cm is suspicious for malignancy until proved otherwise.
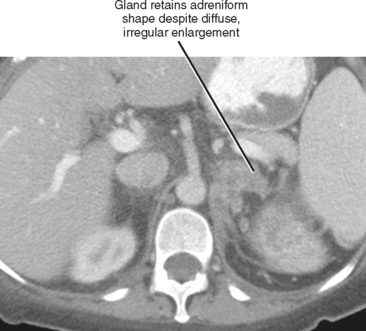
As a rule of thumb, an adrenal limb thicker than 10 mm is considered to be enlarged. Unfortunately, adrenal shape is only occasionally helpful in characterizing adrenal gland enlargement. Diffuse bilateral enlargement of the adrenal glands usually indicates adrenal hyperplasia and can be either smooth or have a nodular contour (Fig. 17-11). Adrenal hyperplasia is associated with clinical findings of Conn or Cushing syndrome in adults and children, as well as virilization in infants. Uncommonly, adrenal metastases can cause diffuse enlargement of the gland (Fig. 17-12), although metastatic enlargement is usually less symmetric than that seen with hyperplasia.
The number of adrenal masses is a weak discriminator between benign and malignant lesions. Adrenal adenomas, carcinomas, metastases, lymphoma, and pheochromocytomas can all present as solitary or multiple adrenal masses. Nodular hyperplasia is easily mistaken for multiple metastatic nodules in a patient at risk for metastatic disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree