Introduction
For both primary and metastatic brain tumors, radiation therapy (RT) remains one of the standard treatment modalities. Although RT techniques using photons have greatly improved in recent decades, substantial concerns remain among both physicians and patients regarding the potential for long-term side effects after RT to the brain.
Adverse effects after RT to the brain are numerous. In consenting when patients give consent for therapy, potential damage to specific structures such as the optic chiasm, optic nerves, or spinal cord is often cited, which could result in vision impairment, paralysis, and so on. Thankfully, such side effects are relatively infrequent. However, subtler long-term side effects may be problematic for both patients and their families. This includes the potential for long-term cognitive deficiencies. Although deficits in memory formation are the most commonly studied in the field of radiation oncology, deficits in attention or other executive functions may be equally as debilitating. Remarkably little is known regarding the biological basis for the side effects. However, progress is being made in the pharmacologic prevention of side effects and with use of advanced photon techniques, such as hippocampal-sparing intensity-modulated RT (IMRT). Proton therapy may offer much greater normal tissue sparing and thereby further decrease the incidence of the side effects.
Particle therapy, including proton therapy, is hoped to expand the therapeutic index of radiation for primary brain tumors. Unlike photons, as the proton beam passes through tissue, protons continuously slow down, and the rate at which they deposit dose increases along their path. The point at which all energy is depleted is termed the Bragg peak. Past the Bragg peak, virtually no extra dose is delivered, and hence, normal tissues distal to the target should receive virtually no radiation exposure. Based on these principles, assuming target volumes are adequately covered, RT with protons should offer equivalent disease control but with superior normal tissue sparing and hence a reduction in long-term adverse effects.
Presently within the United States alone, nearly 30 proton centers are in operation, with many more in the planning or development stage. Although proton therapy has been used in the treatment of a variety of primary brain tumors, no published randomized studies exist to document its clinical superiority in comparison with advanced photon techniques. In the setting of increased scrutiny from insurance providers, this may limit patients’ access to proton therapy. In this review, we highlight the technologies used to deliver proton therapy as well as preliminary clinical studies of primary brain tumors, including gliomas, meningioma, and others. Finally, areas for additional study, including the use of advanced treatment planning techniques, are offered.
Gliomas: Low and high grade
Low-grade gliomas
Gliomas have a broad spectrum of disease types with associated differences in outcomes. Our understanding of the molecular profiles of these tumors continues to evolve rapidly and is now informing us which patients may achieve long-term survival. Mutational profiling is now the standard of care for lower-grade gliomas, including the World Health Organization (WHO) grades II and III. Broadly speaking, patients may be grouped as those having or not having mutations in isocitrate dehydrogenase (IDH). Although a descriptor of grade II or III may be assigned by pathologists, evidence suggests little difference in outcomes based on grade, but the presence of an IDH mutation indicates favorable prognosis. , Conversely for patients with grade II or III tumors where no IDH mutation is identified, these tumors may be classified as a “molecular glioblastoma (GBM),” and patients may experience rapid disease progression similar to that seen with grade IV GBM.
RT has an integral role in the treatment of low-grade gliomas, and although mutational status is now influencing therapeutic decisions, historically, treatment decisions have been based on tumor grade. For WHO grade II gliomas, combined-modality therapy has contributed to improved survival rates, and early RT is associated with improved progression-free survival. , However, the timing of RT that is, whether it is delivered as adjuvant or salvage therapy, remains controversial. The existing controversy centers on the negative effects of radiation on cognitive function and quality of life, which are of special importance in patients with a long life expectancy. For patients with WHO grade III tumors, adjuvant RT is considered standard, although, as noted, mutational profiling allows the prediction of favorable outcomes for subsets of patients. In particular, grade III gliomas with an IDH mutation have favorable outcomes, and numerous patients will achieve long-term survival similar to that seen for patients with WHO grade II tumors. , , As such, these patients are at substantial risk for cognitive decline after RT.
Cognitive decline after cranial irradiation is especially problematic for brain tumor survivors, as it is associated with reduced quality of life. , Historically, the overwhelming majority of radiation treatments have been delivered using photon-based techniques. Douw et al. retrospectively evaluated patients with low-grade gliomas treated with or without RT and found RT use to be associated with impaired attentional functioning and executive function. Gondi et al. prospectively evaluated the effects of radiation on cognitive function in adult patients with low-grade brain tumors treated with advanced photon radiation techniques, including IMRT. The trial included both baseline and postradiotherapy assessments including formal neurocognitive tests. Exposure of the bilateral hippocampi to doses as low as 7.3 Gy was associated with long-term memory impairment.
Such evidence has led practitioners to believe that proton therapy may be ideally suited for the treatment of these tumors. In addition to compelling dosimetric studies, initial clinical studies of proton therapy have suggested efficacy. Investigators from Massachusetts General Hospital (MGH) first used mixed photon/proton treatments for dose-escalation studies including patients with WHO grades II and III gliomas. Investigators from the University of Heidelberg, which uses scanning beam proton delivery technology, have also reported on 19 patients treated for low-grade gliomas. Similar to photon-based treatments, their initial results suggest high rates of tumor control and acceptable toxicity rates. The group at The University of Texas MD Anderson Cancer Center has also reported on outcomes after IMRT or proton therapy for these tumors. Although the latter was a retrospective study, disease control outcomes were similar. However, patients with oligodendroglioma treated with protons developed pseudoprogression sooner than those treated with photons. Formal cognitive testing outcomes were unfortunately not available. In a recent study, Shih et al. reported results of a prospective trial that enrolled patients with grade II gliomas. In addition to reporting excellent disease control rates, they assessed cognitive function and quality of life after proton therapy. Twenty patients, all with supra-tentorial tumors, were enrolled. With a median follow-up time of 5.1 years, measures of cognitive function were stable to improved relative to baseline, with no patients experiencing cognitive failure. Sherman et al. reported that compared with normative practice effects, these patients exhibited less improvement in the domains of processing speed, executive function, and verbal memory. However, because this was an uncontrolled, noncomparative trial, it is unclear if this relative stability reflects the absence of an expected practice effect in this treated population.
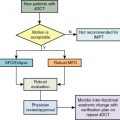
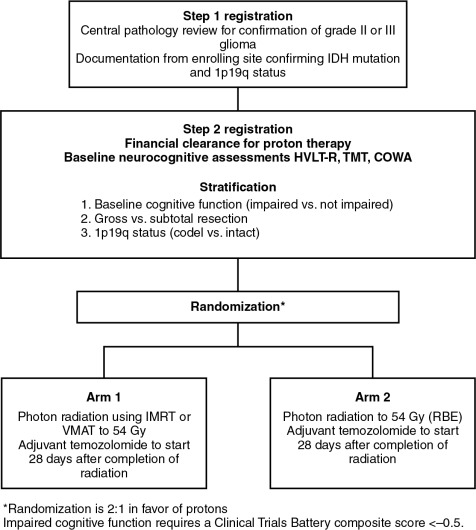
To provide the best evidence supporting the benefits of proton therapy in comparison with the best photon therapy (IMRT), randomized trials are needed. Historically, with a few exceptions, in the field of photon therapy, randomized trials have not been done to compare radiation techniques such as three-dimensional conformal versus IMRT. However, given that proton therapy is a fundamentally different form of radiation, it is not unreasonable that such trials be conducted. Currently, an ongoing randomized phase II trial is being conducted through the NRG Oncology Group, BN005 (clinicaltrials.gov identifier: NCT03180502). The schema for this trial is presented in Fig. 9.1 . Eligible patients include those with an IDH mutant grade II or III glioma and are randomized to receive protons versus photons. The central hypothesis is that the normal tissue-sparing offered by proton therapy will lead to superior preservation of cognitive function and reduced symptom burden relative to patients treated with photon-based therapy.
High-grade gliomas
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor in adults. In contrast to grade II or III glioma, or grade IV glioma with mutations in IDH, GBM has a very poor prognosis. With the current standard postsurgical temozolomide-based chemoradiotherapy, overall median progression-free survival time is approximately 7 months, with overall survival time of 15 months. Despite poor outcomes, even here, trials using proton therapy have been conducted.
Current recommendations for radiation include dosages up to 60 Gy given in daily fractions of 2 Gy to the enhancing area of the tumor with 1- to 2-cm margins. When this standard radiotherapy regimen is used, 80% to 90% of tumors recur within 2 cm of the original lesion. In an effort to improve tumor control, several groups have used proton therapy to escalate doses up to 90 Gy (relative biological effectiveness [RBE]) (Gy[RBE]). MGH treated 23 patients with GBM by using a combination of photons and protons to a total dose of 90 Gy(RBE) to the gross tumor volume, 64.8 Gy to the 2-cm margin encompassing the gross tumor volume, and 50.4 Gy(RBE) to areas of surrounding edema plus 2-cm margins using accelerated fractionation. When patients were stratified by Radiation Therapy Oncology Group (RTOG) prognostic classes, this plan consistently increased median survival time. Dose escalation up to 90 Gy(RBE) yielded median survival times of 23, 17, and 14 months for RTOG classes III, IV, and V, respectively. This compares with the 17.9, 11.1, and 8.9 months median survival time for the respective classes seen in previous RTOG trials using standard doses of RT with chemotherapy. Of the 23 patients, only one patient had tumor recurrence within the dose-escalated region. Despite the good control and increased median survival time, all 7 patients from whom tissue was obtained developed radiation necrosis, and most patients experienced neurological deterioration.
A more recent study from Tsukuba also escalated the dose to 96.6 Gy(RBE) over 56 fractions in 21 patients, most with RTOG class IV GBM, and obtained a median survival time of 21.6 months. When stratified by the size of the enhancing tumor, acute side effects were found to be tolerable for patients with smaller tumor volumes. However, this study could not comment on late effects of the radiation because of the difficulty in distinguishing between tumor recurrence and necrosis on imaging. These studies demonstrate that dose escalation up to 96 Gy(RBE) with proton therapy provides effective local control and promising increases in median survival time.
NRG BN001 also incorporates proton therapy (clinicaltrials.gov identifier: NCT02179086) as part of a national trial. Although no direct randomization is done between protons and photons, BN001 uses either advanced photon or proton therapy to dose-escalate in patients with GBM. Secondary objectives of the trial will include indirect comparisons of the two radiation types in an attempt to determine if dose escalation may be more safely achieved with proton therapy. Notably, a randomized trial of protons versus photons has also been performed and indeed completed in patients with GBM. This was a single-institution trial performed at MD Anderson Cancer Center (clinicaltrials.gov identifier: NCT01854554). Patients with GBM were randomized to protons or photons and underwent serial cognitive testing, with preservation of cognitive function being the primary outcome. The results of this trial have not yet been reported. However, when assessing cognitive dysfunction, the presence of tumor in the brain parenchyma has dramatic negative impacts on cognitive function. Indeed, evidence indicates that higher-grade or IDH nonmutant tumors are more likely to be associated with greater cognitive deficits at baseline.
Meningioma
Meningiomas are the most common benign central nervous system tumors in adults and portend a generally favorable prognosis, as 90% are classified as WHO class I. Surgery is the mainstay of therapy, but radiation is used as adjuvant therapy in cases of partial resections and high-grade or recurrent lesions. RT can also be used as a definitive treatment for lesions in locations where resection is not possible. Long-term control rates with current RT techniques are greater than 90%. Given the expected long-term survival, improving functional status and limiting toxicities are the objectives of treatment.
Given the proximity of skull base meningiomas to critical structures, particle therapy provides an opportunity to reduce toxicities. Wenkel et al. studied 46 patients with benign skull base meningiomas treated with a combination of photon and proton, and reported recurrence-free rates of 100% at 5 years and 88% at 10 years. Four patients in this series experienced ophthalmic toxicity. In retrospect, doses to the optic nerves of these patients were found to exceed the threshold of 54 Gy when doses were recalculated after a recalibration of the particle accelerator. Patients who did not receive >54 Gy to the optic nerve did not experience any ophthalmic toxicity. Neol et al. studied functional outcomes of 51 patients with skull base meningiomas treated with a combination of photons and proton therapy. Four-year local control and overall survival rates were 98% and 100%, respectively. Two patients (3.9%) suffered from grade III side effects. In addition, 68.8% of the eye-related symptoms improved after RT, and 67% of other miscellaneous symptoms improved, which compares favorably with photon studies reporting functional outcomes.
Weber et al. from the Paul Scherrer Institute, which uses a pencil beam scanning–based proton treatment, studied 39 cases treated with only protons as part of RT. At least 10 patients in this series had WHO grade II/III meningiomas, and the average tumor volumes were larger than most other series. Five-year local control and overall survival rates were 84.8% and 81.8% for all histology types and 100% for benign histology. The 5-year grade III/IV toxicity-free survival rate was 84.5%. Patients who experienced late-grade toxicity were those with large tumor volumes and optic tract meningiomas. Initial outcomes seem to support the use of particle therapy for meningiomas, especially for lesions in close proximity to critical structures. However, the study by Wenkel et al. demonstrates the need for careful planning to avoid adverse effects.
Unlike WHO grade I tumors, grade II or III meningiomas, although rare, may be more prone to local recurrence. This has prompted consideration of dose escalation for such tumors. Investigators at MGH have begun enrollment on a prospective study of dose escalation using proton therapy for these aggressive malignancies (clinicaltrials.gov identifier: NCT02693990). As part of this phase I/II trial, intensity-modulated proton therapy (IMPT) for sequential dose escalation for patients with atypical or WHO grade III (malignant) meningiomas.
Pituitary tumors and vestibular schwannomas
Pituitary adenomas are benign tumors found in the sella turcica. RT is typically used after medical and surgical therapies have failed; RT offers the potential for cure, even if the lesion is unresectable. Moreover, medical therapy may require lifelong treatment, or tumors may become refractory to medical management.
Two primary dose schedules are commonly used in RT for pituitary adenomas. Stereotactic radiosurgery (SRS) delivers a high-dose single-radiation treatment (typically 15–20 Gy), whereas fractionated schedules deliver 45 to 54 Gy over 5 to 6 weeks. It has been suggested that SRS normalizes hormone levels of functional adenomas faster than conventional fractionated RT. However, the use of SRS may be limited in tumors located in close proximity to critical structures, such as the optic chiasm, because of the high doses prescribed.
Some institutions have started performing SRS with protons. Similar to fractionated proton therapy, proton SRS (PSRS) exhibits low entry dose and no exit dose, resulting in decreased risk of toxicity. MGH has studied PSRS in the treatment of adrenocorticotropic hormone– and growth hormone (GH)–secreting tumors. A total of 22 patients with residual GH-secreting tumors after transsphenoidal resection were treated using PSRS with a median dose of 20 Gy (RBE). A complete response (CR), defined as sustained (≥3 months) normalization of insulin-like growth factor 1 levels, was seen in 59% of patients after a median of 42 months after radiotherapy. In another study, 38 patients with Cushing disease or Nelson syndrome were treated with PSRS for persistence of symptoms and cortisol levels after transsphenoidal resection. At a median follow-up time of 62 months, CR was achieved in 100% (5 of 5) cases Nelson syndrome (5 out of 5) and in 52% (17 of 33) cases of Cushing disease. The median time to CR was 18 months after PSRS. In both studies, the portion of patients achieving CR and time to CR were comparable with previous SRS studies. Also, no evidence of visual disturbances, seizures, or clinical signs of brain injury were noted. However, both studies experienced slightly higher rates of hypopituitarism after PSRS when compared with other SRS studies. Still, PSRS is a promising RT technique.
Like most pituitary tumors, vestibular schwannomas are benign intracranial tumors. These lesions are believed to arise from the myelin-forming cells of the vestibulocochlear nerve. Observation is a reasonable option for many patients, as many vestibular schwannomas are found only incidentally on imaging, and only 43% to 46% of tumors show any growth, with an average rate of 1.2 to 1.9 mm/year. For tumors requiring treatment, surgery and RT can both be used as first-line treatments. Surgery offers excellent control rates and tends to be used to treat larger tumors with mass effects. Definitive RT is also a therapeutic option that offers excellent tumor control rates of greater than 90%. Although vestibular schwannomas treated with RT may have a reported lower incidence of adverse effects, including hearing loss or facial nerve palsies, compared with microsurgery, direct comparisons are difficult to make because tumors treated with microsurgery tend to be larger.
Harsh et al. used a PSRS protocol prescribing 12 Gy(RBE) to the tumor and limiting the brainstem dose to 12 Gy(RBE) and found control rates of 94%, trigeminal and facial nerve preservation of 95.3%, and hearing preservation of 33.3%. Low rates of hearing preservation were thought to be attributed to the patients being older (mean age = 67 years) and resections being done before RT, which may increase susceptibility to cranial nerve damage. Bush et al. used a fractionated protocol prescribing 54 to 60 Gy(RBE) in 30 to 33 fractions. At a mean follow-up time of 34 months, the control rate was 100%, no trigeminal and facial nerve toxicities were observed, and 31% maintained useful hearing. Using the α-β model to compare doses in fractionated stereotactic RT and SRS studies, the protocol prescribed roughly 40% more radiation at standard fractionation. Prescribing an equivalent dose may have resulted in better-preserved hearing. Vernimenn et al. suggest that hypofractionated proton therapy may also be an option for large, inoperable tumors. The average tumor volume in this series was 5.3 cm , which is among the highest studied. The protocol prescribed 26 Gy(RBE) over 3 fractions and reported a 5-year local control rate of 98%. At a mean follow-up time of 72 months, hearing preservation rate was 42%; trigeminal and facial nerve preservation rates were 93% and 90.5%, respectively. Baummert et al. compared dose distributions of photon and particle therapy and found that conformality was equal, but proton therapy reduced the integral dose. Because greater dose sparing is realized in larger lesions, particle therapy may be particularly useful for larger lesions.
Medulloblastoma and other malignancies
Although more common in pediatric patients, diseases such as medulloblastoma, ependymoma, and craniopharyngioma can also occur in adult patients. For pediatric patients, it is generally assumed that protons will be superior and lead to less long-term adverse effects, as children are likely more sensitive to radiation-induced normal tissue toxicity than adults. For each of the previously listed tumor types, survival outcomes are expected to be good to excellent for adult patients presenting with these tumors. As such, proton therapy is increasingly used for adult patients with these tumors.
For patients treated on the craniospinal axis, such as those with medulloblastoma, data are available to support the notion that proton therapy may have quantifiable benefits. Retrospectively comparing patients treated with photon or protons, Brown at al noted a decreased incidence in nausea and esophagitis with protons. Importantly, patients treated with protons also had better maintained hematologic profiles. With pediatric patients, practitioners typically treat the entire vertebral body to prevent late-growth asymmetries. However, with adults, this is not necessary; rather, in addition to anterior structures such as the heart and thyroid, most of the bone marrow can be spared, likely helping to maintain white blood cell counts. This could be because these patients are likely to receive adjuvant chemotherapy and therefore may better be able to tolerate this treatment.
Diseases such as craniopharyngioma and ependymoma arising in adult patients are rare, and there are few reports of these treated with proton therapy. However, in pediatric patients, these tumors may be successfully treated with protons, and again, the low-dose sparing is expected to translate into a reduction in adverse effects. ,
Radiation techniques and treatment planning
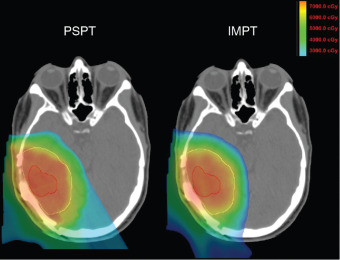
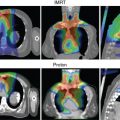
Although the clinical studies reviewed earlier provide initial evidence in support of proton therapy for the treatment of primary brain tumors, most of these patients have been treated with what some may call antiquated proton technology. Similar to advances made in photon therapy in recent decades, proton therapy is a rapidly evolving technology. Since 2010, the number of active centers treating patients, and publishing outcomes, has been small. Moreover, virtually all these centers used passive-scattered proton therapy (PSPT). With PSPT, physical elements are introduced into a broad proton beam to shape the distal and lateral edges. Nearly all new centers now offer only scanning beam proton therapy. With scanning proton therapy, small pristine proton beams are scanned magnetically to conform to the lateral edges of the target volume and energy changes control the depth of penetration. Scanning beam proton therapy allows maximally conformal, not only low-dose, but high-dose radiation ( Fig. 9.2 ). Indeed, in comparing PSPT with IMRT, IMRT plans often may have better high-dose conformity. Scanning beam proton therapy also allows the delivery of true IMPT. With true IMPT, the optimizer has the flexibility to manipulate all beamlets within each proton field simultaneously to deliver a maximally conformal treatment plan. This review of the literature should account for the fact that most patients were treated with what may be considered first-generation proton therapy delivery techniques in which the normal tissue sparing in high-dose regions is not maximized.