F-sodium fluoride ( F-NaF) PET/CT studies. We first segment the cortical shell of the vertebral body and unwrap it to a 2D map. Multiple characteristic features derived from PET/CT images are then projected onto the map. Finally, we adopt a three-tier learning based scheme to compute a confidence map and detect osteophyte sites and clusters. The system was tested on 20 studies (10 training and 10 testing) and achieved 84 % sensitivity at 3.8 false positives per case for the training set, and 82 % sensitivity at a 4.7 false positive rate for the testing set.
F-NaF) PET/CT studies. We first segment the cortical shell of the vertebral body and unwrap it to a 2D map. Multiple characteristic features derived from PET/CT images are then projected onto the map. Finally, we adopt a three-tier learning based scheme to compute a confidence map and detect osteophyte sites and clusters. The system was tested on 20 studies (10 training and 10 testing) and achieved 84 % sensitivity at 3.8 false positives per case for the training set, and 82 % sensitivity at a 4.7 false positive rate for the testing set.
1 Introduction
Degenerative disc disease (DDD) develops with degeneration of the nucleus pulposus of the intervertebral discs (IVD) of the spine. As the nucleus pulposus desiccates, its volume, height, and elasticity are reduced and the IVD loses its ability to stably support loads. Spinal osteophytes are abnormal bony outgrowths that form along the disc margins in response to degenerative changes in the IVD and the associated altered biomechanics between the vertebral bodies. This osteophyte development occurs at the intervertebral interspaces and can inhibit normal spinal motion as well as progress to complete osseous bridging that fuses vertebrae. Osteophytes become more prevalent in the spine with increasing age, and are found in 90 % of population over 60 years old [1].
Investigations into computer-aided evaluation of spinal pathology and condition have been limited. The main areas of focus to date have involved spine lesions, scoliosis, fractures, and morphological change. There have been a few prior works targeting degenerative change and spinal osseous excrescences. Tan et al. [2] sought to quantitatively measure the status of ankylosing spondylitis via the segmentation of individual vertebra with successive level sets, followed by the segmentation of bony outgrowths (syndesmophytes) and quantification of their volume and height. Another method [3] dealt directly with detection of osteophytosis in the spine using radiographs of the cervical spine to detect and classify types of anterior osteophytes. Herrmann et al. [4] tracked the time variation of vertebral morphology between radiographs due to degenerative changes. While these methods focus on osseous excrescences which can be secondary indicators of DDD, other methods have focused directly on the IVD themselves. One method detected degenerating IVDs on MRI using 2D methods that analyzed disc intensity, location, and spacing [5]. A more recent approach segmented both the vertebral bodies and IVDs to detect degenerating IVDs in asymptomatic patients [6].
Degenerative osteophytes present in a variety of sizes, shapes, and densities, some examples of which are shown in Fig. 1, and can sometimes mimic the appearance of other pathologic processes. Osteophytes can be differentiated,in part, from dense regions of the spine of alternative etiology, by their spatial localization to the cortical shell of vertebra. They often form oblique longitudinal patterns across many vertebral bodies, following the distribution of biomechanical load stressors as modified by physiologic homeostatic processes. CT imaging is useful to detect these osteophytes, diagnosed as marginal regions of dysmorphic cortical shell thickening of the vertebral bodies, typically (but not universally) with a higher X-ray attenuation than the adjacent cortex. Additionally, on physiologic imaging modalities, osteophytes may manifest with increased activity due to processes such as active mineralization, induced by mechanical stressors and associated progressive exostosis. Unfortunately, actively mineralizing bone, which has preferential uptake in  F-sodium fluoride (
F-sodium fluoride ( F-NaF) PET can be found in both osteophytes and metastases. Thus, osteophytes and spine metastases can manifest with similar and overlapping appearances on CT and
F-NaF) PET can be found in both osteophytes and metastases. Thus, osteophytes and spine metastases can manifest with similar and overlapping appearances on CT and  F-NaF PET images. Our goal is to explore the set of multimodal imaging features of osteophytes on PET and CT, characterizing both physiological and morphological elements, to search for and incorporate synergistic combinations of these features into a CAD system that can automatically detect spinal osteophytes on
F-NaF PET images. Our goal is to explore the set of multimodal imaging features of osteophytes on PET and CT, characterizing both physiological and morphological elements, to search for and incorporate synergistic combinations of these features into a CAD system that can automatically detect spinal osteophytes on  F-NaF PET/CT.
F-NaF PET/CT.
 F-sodium fluoride (
F-sodium fluoride ( F-NaF) PET can be found in both osteophytes and metastases. Thus, osteophytes and spine metastases can manifest with similar and overlapping appearances on CT and
F-NaF) PET can be found in both osteophytes and metastases. Thus, osteophytes and spine metastases can manifest with similar and overlapping appearances on CT and  F-NaF PET images. Our goal is to explore the set of multimodal imaging features of osteophytes on PET and CT, characterizing both physiological and morphological elements, to search for and incorporate synergistic combinations of these features into a CAD system that can automatically detect spinal osteophytes on
F-NaF PET images. Our goal is to explore the set of multimodal imaging features of osteophytes on PET and CT, characterizing both physiological and morphological elements, to search for and incorporate synergistic combinations of these features into a CAD system that can automatically detect spinal osteophytes on  F-NaF PET/CT.
F-NaF PET/CT.
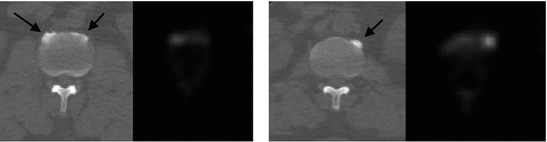
Fig. 1
Examples of osteophytes (arrows) on axial CT (left) and PET (right) images at two different vertebral levels
2 Methods and Material
Data: With IRB approval, we collected 20  F-NaF PET/CT scans from 20 patients. The study population consisted of 15 males and 5 females, with a mean age of
F-NaF PET/CT scans from 20 patients. The study population consisted of 15 males and 5 females, with a mean age of 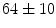 . All patients were scanned on a Philips GEMINI TF scanner. Doses ranging from 112
. All patients were scanned on a Philips GEMINI TF scanner. Doses ranging from 112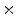 10
10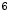 to 203
to 203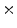 10
10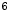 Bq/ml of
Bq/ml of 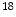 F-NaF were administered intravenously to the patients, followed by physiologic uptake periods ranging from 114 to 126 min prior to image acquisition. The axial PET images were 144
F-NaF were administered intravenously to the patients, followed by physiologic uptake periods ranging from 114 to 126 min prior to image acquisition. The axial PET images were 144 144 or 169
144 or 169  169 pixels, at an axial spatial resolution of 4 mm
169 pixels, at an axial spatial resolution of 4 mm 4 mm per pixel and 4 mm slice spacing. Corresponding low dose technique of CT scanning was also performed. The scanning parameters for CT were: 5 mm slice thickness, 120 kVp, no intravenous contrast administration, and convolution kernel B. An experienced radiologist manually annotated the location of osteophyte sites on each CT slice (shown in Fig. 4).
4 mm per pixel and 4 mm slice spacing. Corresponding low dose technique of CT scanning was also performed. The scanning parameters for CT were: 5 mm slice thickness, 120 kVp, no intravenous contrast administration, and convolution kernel B. An experienced radiologist manually annotated the location of osteophyte sites on each CT slice (shown in Fig. 4).
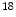 F-NaF PET/CT scans from 20 patients. The study population consisted of 15 males and 5 females, with a mean age of
F-NaF PET/CT scans from 20 patients. The study population consisted of 15 males and 5 females, with a mean age of  . All patients were scanned on a Philips GEMINI TF scanner. Doses ranging from 112
. All patients were scanned on a Philips GEMINI TF scanner. Doses ranging from 112 10
10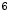 to 203
to 203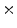 10
10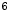 Bq/ml of
Bq/ml of 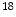 F-NaF were administered intravenously to the patients, followed by physiologic uptake periods ranging from 114 to 126 min prior to image acquisition. The axial PET images were 144
F-NaF were administered intravenously to the patients, followed by physiologic uptake periods ranging from 114 to 126 min prior to image acquisition. The axial PET images were 144 144 or 169
144 or 169  169 pixels, at an axial spatial resolution of 4 mm
169 pixels, at an axial spatial resolution of 4 mm 4 mm per pixel and 4 mm slice spacing. Corresponding low dose technique of CT scanning was also performed. The scanning parameters for CT were: 5 mm slice thickness, 120 kVp, no intravenous contrast administration, and convolution kernel B. An experienced radiologist manually annotated the location of osteophyte sites on each CT slice (shown in Fig. 4).
4 mm per pixel and 4 mm slice spacing. Corresponding low dose technique of CT scanning was also performed. The scanning parameters for CT were: 5 mm slice thickness, 120 kVp, no intravenous contrast administration, and convolution kernel B. An experienced radiologist manually annotated the location of osteophyte sites on each CT slice (shown in Fig. 4).2.1 Method Overview
For a given PET/CT data set, the PET data is first resampled to have the same resolution as the CT data. The spine is segmented on the CT images. The cortical shell of vertebral body is then extracted and unwrapped to a 2D map. Morphological and physiological features derived from both CT and PET are projected onto the map. A three-tier classification scheme is then applied to detect spinal degenerative osteophytes. The annotated location markers for the osteophytes are used as the reference standard to train the classifiers at each stage.
2.2 Spinal Segmentation and Cortical Shell Unwrapping
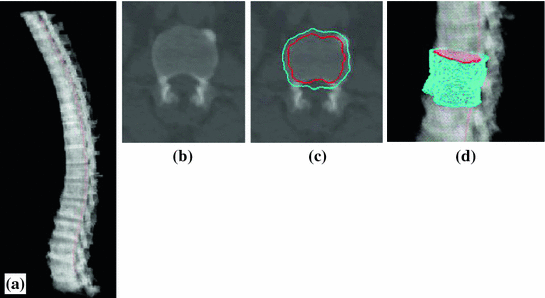
Spine segmentation is accomplished through thresholding, fuzzy connectivity and anatomical vertebral models. The spinal canal is first extracted using a directed graph search. Then a vertebral template is fit along the spinal canal. Finally, the spinal column is partitioned into vertebrae by detecting the IVD on curved planar reformations in sagittal and coronal directions. Details of the automated spinal column extraction and partitioning can be found in [7]. Figure 2a shows the segmented spine.
Since degenerative osteophytes occur at the cortical shell of the vertebral body, we apply a deformable dual-surface model to extract the periosteal and endosteal surfaces of the cortical shell at each vertebral level. The initial model consists of two concentric cylinders with their axes aligned with vertebral body axis and radii twice the average radius of vertebral bodies. The surface is represented as 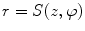 in the cylindrical coordinate system, where z is the distance along the axis,
in the cylindrical coordinate system, where z is the distance along the axis, 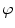 is the azimuth angle, and r is the radial distance. The vertices on the surface are then denoted as (
is the azimuth angle, and r is the radial distance. The vertices on the surface are then denoted as (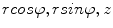 ). The periosteal and endosteal surfaces are represented as
). The periosteal and endosteal surfaces are represented as 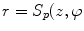 ) and
) and 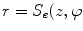 ) respectively. The dual-surface is driven by the synergy of internal force, image force and constraint between the two surfaces, written as,
) respectively. The dual-surface is driven by the synergy of internal force, image force and constraint between the two surfaces, written as,
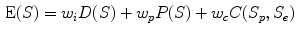
where the internal forces D(S) are spline forces derived from partial differential geometry that keep the surface smooth and continuous. The potential forces P(S) are derived from the directional gradient along the radial direction of the cylindrical coordinate system. The dual-surface constraint ensures thickness transitions smoothly over the cortical shell, written as,
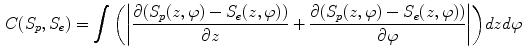
The weights for different forces (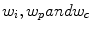 ) in Eq. 1 are kept constant throughout the evolution. Figure 2b-d shows the result of cortical shell segmentation.
) in Eq. 1 are kept constant throughout the evolution. Figure 2b-d shows the result of cortical shell segmentation.
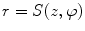 in the cylindrical coordinate system, where z is the distance along the axis,
in the cylindrical coordinate system, where z is the distance along the axis, 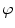 is the azimuth angle, and r is the radial distance. The vertices on the surface are then denoted as (
is the azimuth angle, and r is the radial distance. The vertices on the surface are then denoted as (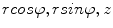 ). The periosteal and endosteal surfaces are represented as
). The periosteal and endosteal surfaces are represented as 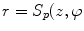 ) and
) and 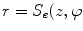 ) respectively. The dual-surface is driven by the synergy of internal force, image force and constraint between the two surfaces, written as,
) respectively. The dual-surface is driven by the synergy of internal force, image force and constraint between the two surfaces, written as,
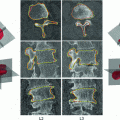
Fig. 2
Spine and cortical shell segmentation. a Spine segmentation result. Red line: centerline of spinal canal; b one cross section of the vertebra; c cortical shell segmentation at one cross section, cyan: periosteal surface, red: endosteal surface. d 3D surface of the cortical shell segmentation
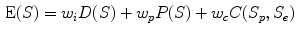
(1)
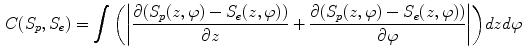
(2)
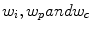 ) in Eq. 1 are kept constant throughout the evolution. Figure 2b-d shows the result of cortical shell segmentation.
) in Eq. 1 are kept constant throughout the evolution. Figure 2b-d shows the result of cortical shell segmentation.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree