Country
Year
Host
Poland
1995–2006
Fox
1955–2007
Human
Slovenia
2006–2007
Human
Hungary
1988
Human
2003, 2008–2009
Fox
Slovakia
1999, 2001
Fox
2009
Dog
2000–2008
Human
Bulgaria
1980
Rodents
Turkey
1934, 1939
Human
1965
Fox
Greece
1978
Human
Moldavia
1961
House mouse
1971
Fox, raccoon dog, human
Ukraine
1957, 2006–2008
Fox
Romania
2009, 2011
Fox, voles, human
Armenia
1958
Human
Belarus
1957, 1958
Rodents
2001, 2003
Fox
Lithuania
2003, 2009
Beaver
2005–2006
Dog, pig
1997–2006
Human
2001–2004
Fox
Latvia
2008
Fox
Estonia
2005
Fox
Russia
1957
Fox
1966
Wild cat
1970, 1972, 1998
Fox, human
Kyrgyzstan
2010
Fox
The main source of infection and life cycle in Europe is forestry including mainly the European red fox (Vulpes vulpes) and several species of rodents as intermediate hosts: water mouse (Arvicola terrestris), field mouse (Microtus arvalis), forest mouse (Clethrionomys glareolus), and other species. Foxes can spread oncospheres of tapeworm in an area over 18 ha and at distances over 15–16 km. Also, from a single stool containing oncospheres, in 10 days they can disperse distances of over 80 m from the excrement in question. Increasing fox populations and extending their life by urban territories is an increased risk factor for humans, and this is all the more so as these animals, the definitive hosts, show no clinical signs to a massive 100,000 tapeworm infection. The E. multilocularis oncospheres are viable outdoors 3–8 months during summer and autumn and in cold temperatures in winter up to 8 months.
Foxes and small rodents represent the natural hosts of parasite. Naturally the parasite transmits between foxes or dogs and small mammals, while humans are aberrant intermediate hosts. Transmission of AE to humans is by consumption of parasite eggs which are excreted in the feces of the definitive hosts: foxes and, increasingly, dogs (Sikó 2011a). Human infection can be through direct contact with the definitive host or indirectly through contamination of food and contaminated green vegetables and fruits or possibly water with parasite eggs.
The incubation period is long (5–15 years); the parasitic tissue grows slowly and is of the infiltrating type. The liver is the organ primarily involved. Metastasis may occur and can involve virtually any organ. In systematic surveys, metastasis to the brain was reported in only 1–3 % of patients with AE (Bresson-Hadni et al. 2000; Kern et al. 2003). Among possible metastases of hepatic AE, locations to the brain are rare, usually fatal, and they have especially been assigned to concomitant immune suppression (Yang et al. 2005; Tappe et al. 2008). Brain metastasis is considered a sign of the terminal phase of the disease (Barjhoux et al. 1970; Bresson-Hadni et al. 2000). Few reports on the rare primary infections of the brain have been published (Aydin et al. 1986; Qiu et al. 1986). Pregnancy has been proposed to play a role as a predisposing factor for cerebral metastasis in AE (Yang et al. 2005).
Pathophysiology and Pathogenesis
AHD is very aggressive. Lesions develop rapidly in the liver where an invasive pseudomalignant (paraneoplastic) mass may be produced. This may spread locally to adjacent organs such as the lung or metastasize to distant structures (Sikó 2011b).
According to the results of animal experiment in Japan, the mechanism of metastasis was due to the detached proliferated bud, even very small or only a few nuclei entering the blood vessels (Matsuhisa et al. 1996). Nevertheless, it was incapable of causing metastasis if protoscoleces were injected into the vessels.
In the European registry 34 % of patients who have metacestode lesions in the liver have simultaneously a lesion in one or more extrahepatic organs, at the time when the patients reach medical attention (Kern et al. 2003).
The metacestode was characterized by internally protrusive hyperplasia from the mother alveolar wall into the alveolar cavity and then proliferation extending continuously to reach the opposite wall of the cavity. So, septum-like budding was formed. Sometimes two or more proliferative sites on the alveolar wall propagated simultaneously into the cavity in opposite directions and mingled with each other to form a septum dividing the mother cyst to form two or more small alveoli (Jiang et al. 2005).
After ingestion of contaminated foods and/or water, embryos of E. multilocularis migrate through the portal system to the liver where vesicular masses are formed. The disease may extend gradually to adjacent organs or spread via the bloodstream (hematogenous metastasis) to distant areas such as the brain. Primary affected is usually the liver, and then the secondary localization by metastasis is in the lung. Bensaid et al. (1994) report a patient with right frontal lobe and palpebral lesions secondary to a primary hepatic focus with secondary lesion in the lung. The intracerebral, frontal lobe, palpebral lobe, disseminated, or other CNS localizations appeared usually after the lung metastasis due to an immunosuppressive effect of parasite.
Sato et al. (1998) made an experimental infection of larval E. multilocularis in the rodent brain attempting to establish a murine model for cerebral AE. Mice and jirds (Meriones genus) were injected intracranially with 10 % of a homogenated hydatid cyst mass. Small cystic larvae were observed macroscopically in the cranial cavity 1, 2, and 5 months post infection in both mice and jirds. Some larval cysts from both rodents contained mature or immature protoscoleces. In mice, the laminated layer was found in the lateral ventricle 2 months post infection but without protoscoleces. At 5 months post infection, larger larval cysts were found in the cranial cavity of a mouse, which also demonstrated partial palsy of the legs. A laminated layer with mature protoscoleces was observed in the third ventricle, and the mouse also harbored, in the left lung, a larval cyst containing protoscoleces surrounded by lymphocytes. Jirds were also found to be infected with metacestodes in the cranial cavity, but neither unusual behavior nor establishment of cysts inside the brain was observed in jirds during the course of infection.
Clinical Features
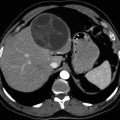
In the cerebral AE the lesions may remain asymptomatic until they are quite large. The clinical presentation usually includes symptoms of increased intracranial pressure, progressive and severe headaches (vomiting, motor weakness, etc.), seizures, cranial nerve findings, and visual disturbances (Takci et al. 2008). Ozdol et al. (2011) present a case of a 23-year-old man with nausea, imbalance, occasional urinary and fecal incontinence, and a severe headache for 1 month. These symptoms were caused by multiple calcified mass lesions in the right upper lobe of the lung and right liver and another solid tumor between the right kidney and liver. Other symptoms such as hemiparesis, seizures, visual field alteration, and gait disorders may vary with the location of the metacestode. Cerebral AHD can also cause intracranial hypertension, seizures, and focal neurological deficits. However, the clinical course is usually more rapid and more severe than in cystic hydatid disease. AE cases are usually present in adults. The average age of patients with cerebral AE is significantly higher than the age of those with cystic echinococcosis (Ammann and Eckert 1996).
Types of Lesions
The infiltrating growth of E. multilocularis metacestode is particularly significant because it may resemble a neoplasm, the so-called malignant hydatid disease. It is especially serious when the E. multilocularis lesions involve the brain, termed a “fatal disease” (Qiu et al. 1986). Cerebral metastasis of primary hepatic AE is often multifocal (Tappe et al. 2008). The cerebral AE is present like multiple intracranial masses which show cauliflower-like contrast enhancement pattern on magnetic resonance imaging (MRI) (Tunaci et al. 1999).
Based on imaging characteristics the AE lesions are divided into three types: (1) solid type (a solid lesion without liquid necrosis or only small patches of necrosis), (2) mixed type (solid component surrounding large and/or irregular liquid necrosis area), and (3) pseudocystic type (large cyst without visible solid component).
Calcification in alveolar echinococcosis may be categorized into three patterns: (1) mild calcification (inconspicuous calcification or punctuate scattered calcification), (2) moderate calcification (coastline calcification located at the periphery of the lesion, with or without the central dot-calcification), and (3) abundant calcification (large calcified deposits) (Wang et al. 2011).
According to the Chinese records, intracranial AE can be subdivided into three types: (a) monomassive type, (b) multifocal type, and (c) extracerebral type (Qiu et al. 1986). The volumes of the lesions are variable, between 6.0 × 4.5 × 3.5 cm3 and 9.0 × 6.5 × 8.5 cm3 (Qiu et al. 1986).
The most frequent morphological profile of AE is represented by a heterogeneous, infiltrative, and destructive mass (Fig. 24.1), with irregular outlines and a nonvascular and necrotic center. The cyst it appears as a spongy structure, resembling a crumb of cheese, with empty cavities or with a gray jelly content (Fig. 24.2). Most of the cavities do not have a well-defined content. In other samples, the formation had mainly a cystic structure, without a divided multivesicular character. In the intracerebellar tissues the metacestode showed a pale yellow mass. Calcification and perilesional edema are prominent (Algros et al. 2003).
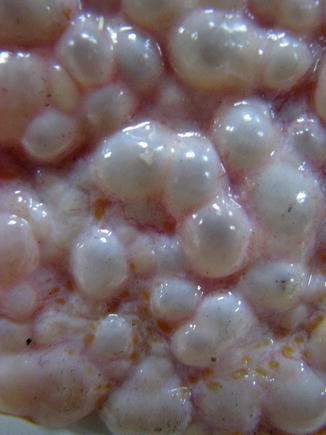
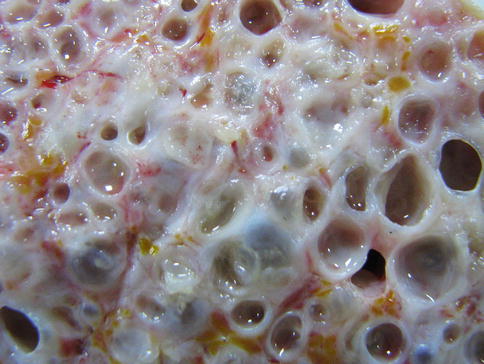

Fig. 24.1
Alveolar echinococcosis an expansive tumoral formation

Fig. 24.2
The internal spongy structure of the tumoral formation
In contrast with E. granulosus, the E. multilocularis cysts are small, group in clusters, elicit a severe inflammatory reaction from the host, and tend to metastasize both locally and distantly. Within the CNS, they usually are located within the brain parenchyma. Intracranial AE is thought to be rare with CNS involvement usually a manifestation of metastases from a primary lesion.
AHD is a biologically aggressive infestation mimicking a malignant neoplasm as assessed radiologically and macroscopically (Ozdol et al. 2011). AE develops within the liver as a rapid invasive pseudomalignant growth and may metastasize to different organs. AE involvement of the brain and liver is similar to an infiltrative tumor (Sikó 2011a, b).
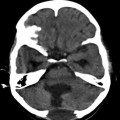
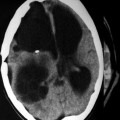
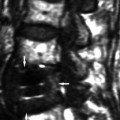
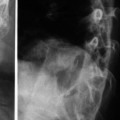
Cerebral cystic echinococcosis lesions are usually single. The cerebral alveolar echinococcosis lesions may be single (and multiseptated) or multiple (Tunaci et al. 1999). Piotin et al. (1997) reported a disseminated intracerebral AE case, secondary to primary hepatic infection. Oktar et al. (1999) described a brain computed tomography (CT) which showed multiple intracranial lesions with remarkable peritumoral cerebral edema. Hakan and Aker (2001) describe a 53-year-old man who presented with headache and right-sided seizures, and on MRI multiple cranial lesions were seen (Figs. 24.3 and 24.4). There were three multiloculated cystic lesions, in the left cerebellum, in the right occipital and left frontal lobes, and two solitary lesions, in the left frontal and left parietal lobes. CT showed multiple masses in the liver and lung. Aydinli et al. (2008) reported a 62-year-old man who had liver AHD with simultaneous lung and brain metastases. Other intracranial localizations were reported in the right frontal lobe and palpebral lesions secondary to a primary hepatic focus with secondary lesion in the lung (Bensaid et al. 1994).
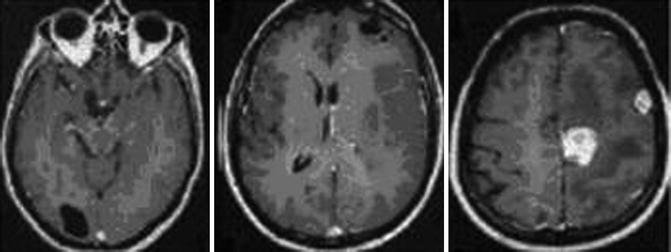
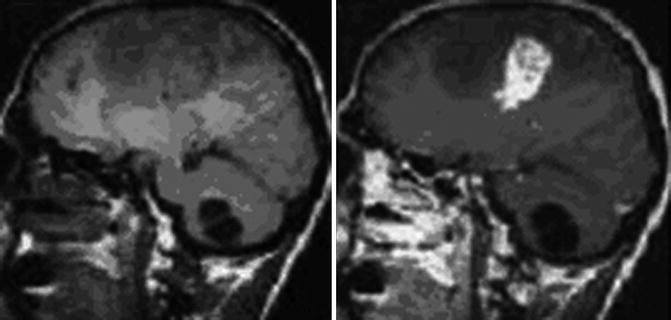

Fig. 24.3
Sagittal T1-weighted MRIs with a subcortical, solitary multilocular cystic lesion (From Hakan and Aker (2001), with permission)

Fig. 24.4
Axial CT of the head after contrast administration. The multiloculated cystic lesions in right occipital, in left frontal lobe, and solitary lesions, one in left frontal and the other one in left parietal lobe (From Hakan and Aker (2001), with permission)
The histopathological examination reveals a diffuse growth composed of compartments that are filled with a gelatinous matrix and many brood capsules and protoscoleces filled with necrotic tissue (Sikó 2011a, b). The larval cestodes of E. multilocularis produce a typical infiltration and induce destruction of the organ. The human organism responds with a granulomatous inflammatory reaction and peripheral formation of granulation tissue. In centripetal direction, the adventitial layer’s components are formed by the fibroconjunctive reaction; inflammatory cells are present. In the peripheral areas lymphocytes and monocytes dominate. They are followed by a rich infiltration of the giant cells and a decrease of the inflammatory local reaction (Fig. 24.5). These structures were gradually replaced by a fibroconjunctive reaction. The surrounding brain tissues show edema, degeneration of neurons, proliferation of gliocytes, and an increase in the number of vessels with distension of lumen by engorgement and complicated by focal necrosis (Qiu et al. 1986; Sikó 2011a, b).
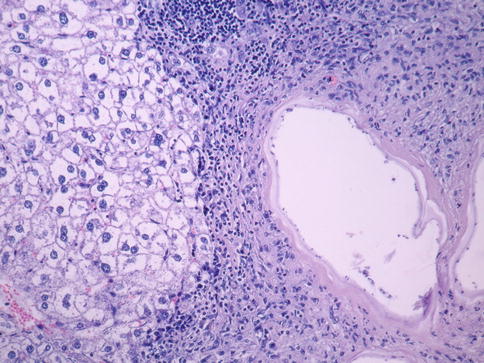

Fig. 24.5
Inflammatory local reaction with multinucleate giant cells, lymphocytes, plasmocytes, and macrophages. Hematoxylin-eosin stain, ×200
Poorly hyalinized cuticle and more cellular reaction, especially with multinucleated giant cells, could be seen in the dense fibrous tissue infiltrated by an inflammatory cell layer. The cuticular layer is mono- or multistratified, formed by concentric conjunctival tissue layers, which penetrate in the cystic cavity and develop many anastomoses conferring it a multivesicular aspect. In the spaces between these blade cells similar to giant cells, we could also notice rare histiocytes and eosinophils. Due to the multitude of anastomoses, the cyst is a structure with an invasive character (Fig. 24.6). In most cases there were collapsed cysts characterized by a fragmented or folded laminar layer and massive necrosis.
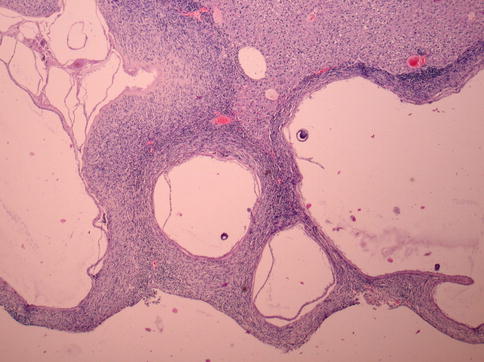

Fig. 24.6
Anastomosis with an invasive character. Pappenheim’s stain, ×40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree