Fig. 1
CT angiogram showing an independent origin from the abdominal aorta of the common hepatic artery, the left gastric artery, and the splenic artery. This information is useful prior to performing a DSA study
2 Liver Arterial Anatomy
2.1 Classical Description
In the classical description of the arterial anatomy, the celiac trunk trifurcates into the left gastric, splenic, and common hepatic arteries. The common hepatic artery divides into the gastroduodenal artery and proper hepatic artery. This itself bifurcates into a right and left branch to supply each of the lobes of the liver. From this point onward, the arterial branching pattern follows the segmental anatomy of this organ and hence the right hepatic artery further divides into the right anterior and posterior hepatic arteries, while the left hepatic artery divides into branches supplying segment II and segment III. The arterial supply to segment IV may occur from one or more branches arising from the right, left, or proper hepatic artery. However, this classical description of the hepatic arterial anatomy occurs in only 55–65 % of the population. Any hepatic arterial anatomy that differs from what has been so far described is considered to represent an anatomical variation.
2.2 Terminology
Quite often, the hepatic artery has an incomplete set of branches because one or the other of its usual branches arises from a source other than the proper hepatic artery from the celiac trunk. Such a vessel if from an outside source is spoken of as ‘aberrant’ (a variation). Aberrant hepatic arteries are of two types, replacing and accessory (Fig. 2). An aberrant hepatic artery refers to a branch that does not arise from its usual source (i.e., proper hepatic artery from the celiac trunk). This type of artery may be a substitute for the usual hepatic artery that is absent, in which case it is referred to as an aberrant ‘replaced’ hepatic artery. In other cases, there may be an additional artery to the one normally present; hence, the term aberrant ‘accessory’ artery.
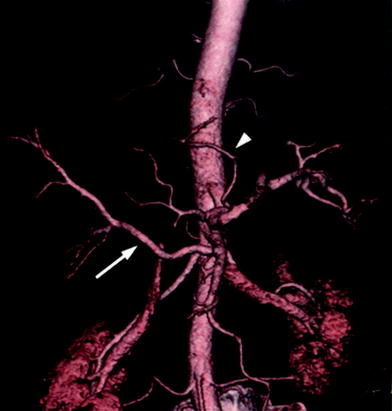

Fig. 2
Aberrant replaced right hepatic artery coming of the superior mesenteric artery (arrow) and aberrant accessory left hepatic artery (arrowhead) coming of the left gastric artery
2.3 Michels’ Classification of Anatomical Variants
The first published description of aberrant hepatic arteries is attributed to Haller in 1756. The data necessary for the study of such variations may be obtained from direct observation of large autopsies, surgical series, or from radiological studies, initially by conventional angiography including DSA or more recently from CT or MR angiography.
In 1953, Michels published his classical study of hepatic arterial anatomy, which detailed the results following the dissection of 200 cadavers (Michels 1953).
He defined 10 anatomical variations of the hepatic artery (Table 1). Later on in 1966, Michels proposed an internationally recognized classification of these hepatic abnormalities, which was later, modified by Hiatt in 1994 (Hiatt et al. 1994).
Table 1
Relevant anatomic variants described by Michels
Type I | Standard (55 %) |
Type II | Replaced LHA (10 %) |
Type III | Replaced RHA (11 %) |
Type IV | Replaced RHA and LHA (1 %) |
Type V | Accessory LHA from LGA (8 %) |
Type VI | Accessory RHA from SMA (7 %) |
Type VII | Accessory RHA and LHA (1 %) |
Type VIII | Accessory RHA and LHA and replaced RHA or LHA (2 %) |
Type IX | CHA replaced to SMA (4.5 %) |
Type X | CHA replaced to LGA (0.5 %) |
In the conventional anatomy defined as type I according to Michels’ classification (Fig. 3), the main hepatic artery originates from celiac trunk, gives off the gastroduodenal artery and the proper hepatic artery, the proper hepatic artery continues as the right hepatic artery after giving off the left hepatic artery and then the right hepatic artery splits into its anterior and posterior branches. The left hepatic artery splits into branches that feed segments II and III. Segment IV is fed by the branch or branches originating from the right, left, or the proper hepatic artery. The left hepatic artery originating from the left gastric artery (replaced left hepatic artery) is defined as type II (Fig. 4), the right hepatic artery stemming from the superior mesenteric artery (replaced right hepatic artery) as type III (Fig. 5), and coexistence of both situations is defined as type IV. The left lobe is also fed by the accessory left hepatic artery originating from the left gastric artery in type V variation, and the right lobe is also fed by accessory right hepatic artery originating from the superior mesenteric artery in type VI variation. Both the left and right accessory artery exist in type VII; the replaced right hepatic artery and the accessory left hepatic artery or the accessory right hepatic artery and the replaced left hepatic artery coexist in type VIII. The hepatic trunk originates from the superior mesenteric artery in type IX and from the left gastric artery in type X.
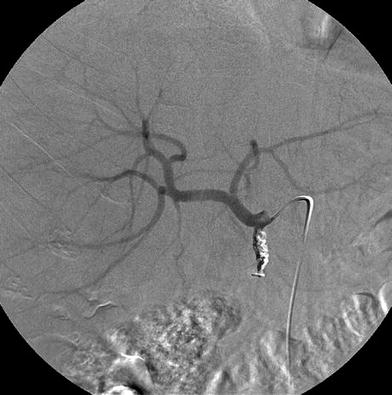
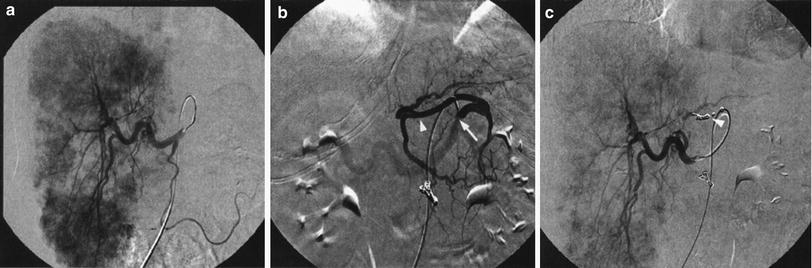
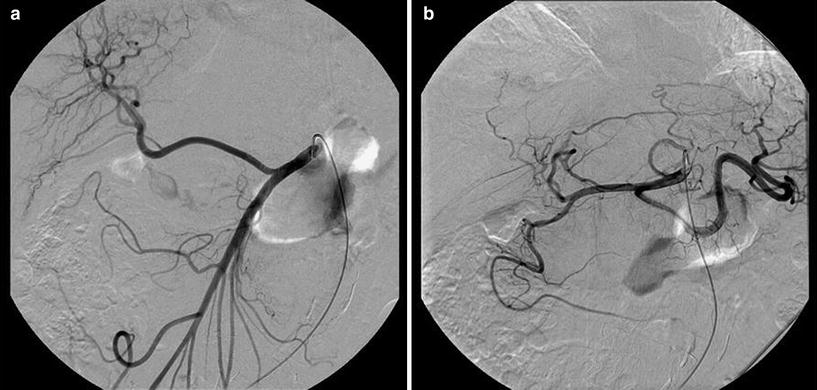

Fig. 3
Conventional anatomy defined as type I according to Michels’ classification. The main hepatic artery originates from celiac trunk, gives off the gastroduodenal artery (which has been embolized with coils) and the proper hepatic artery, this later continues as the right hepatic artery after giving off the left hepatic artery and then the right hepatic artery splits into its anterior and posterior branches. The left hepatic artery feeds segments II and III. Segment IV is fed by the branch or branches originating from the right, left, or the proper hepatic artery

Fig. 4
a Absence of opacification of the left lobe of the liver following injection of contrast in the common hepatic artery. b Injection in the left gastric artery (arrow) shows filling of a replaced left hepatic artery (arrowheads) (type II in Michels’ classification). c Following coil embolization of the origin of the left hepatic artery from the left gastric artery (arrowhead) there is immediate opacification of the whole liver from the common hepatic artery allowing for whole liver treatment from a single injection site

Fig. 5
Type III anatomic variant according to Michels’ classification. Replaced right hepatic artery originating from the superior mesenteric artery
2.4 Other Anatomical Variants
The reported prevalence of anomalies not included in Michels’ system varies from 1.8 (Koops et al. 2004) to 16.6 % (Coskun et al. 2005). The knowledge of these aberrant vessels can be useful in radioembolization, in order to recognize liver parenchyma supplied these arteries and if necessary embolize them before treatment.
Additional clinically relevant anatomical variants are summarized in Table 2 (Figs. 6, 7, and 8).
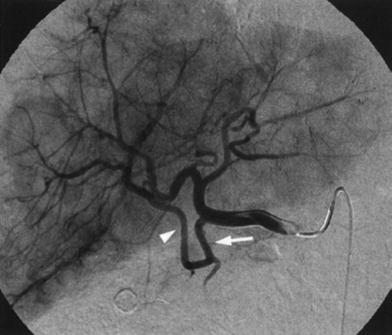
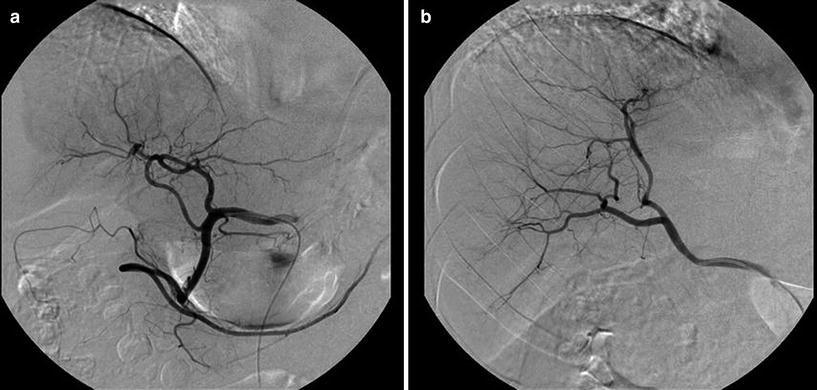
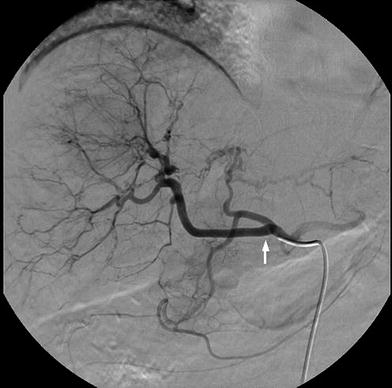
Table 2
Relevant anatomic variants not described by Michels
CHA from aorta |
LHA from LGA + RHA from CHA |
CHA from aorta + aberrant LHA from LGA + aberrant RHA from SMA |
LHA from CHA + RHA from GDA |
CHA from CT + aberrant LHA from LGA + aberrant RHA from aorta |
Celiac mesenteric trunk + LHA from LGA |
RHA from GDA |
LHA from CHA + RHA from SMA |
RHA from CT |

Fig. 6
Another rare variant not described by Michels, consisting in a segmental right hepatic artery (arrowhead) originating from the gastroduodenal artery (arrow)

Fig. 7
Anatomical variant not included in Michel’s system. a Aberrant accessory left hepatic artery arising from the gastroduodenal artery and b aberrant replaced right hepatic artery arising from the superior mesenteric artery

Fig. 8
A rare anatomical variant consisting in the origin of the right hepatic artery (arrow) from the common hepatic artery
3 Extrahepatic Vessels Originating from the Hepatic Vasculature
When performing a radioembolization procedure it is of utmost importance to identify and isolate any vessel which may supply blood to other organs than the liver as this may result in non-target 90Y administration. This is one of the most serious complications, particularly when it happens to affect the GI tract, as it will invariably lead to severe gastritis and possibly even ulceration (Carretero et al. 2007).
Small, previously unseen vessels can become more prominent after embolization of the gastroduodenal and right gastric arteries. If this redistribution phenomenon is not recognized at the time of treatment, complications may ensue (Lewandowski et al. 2007) (Fig. 9).
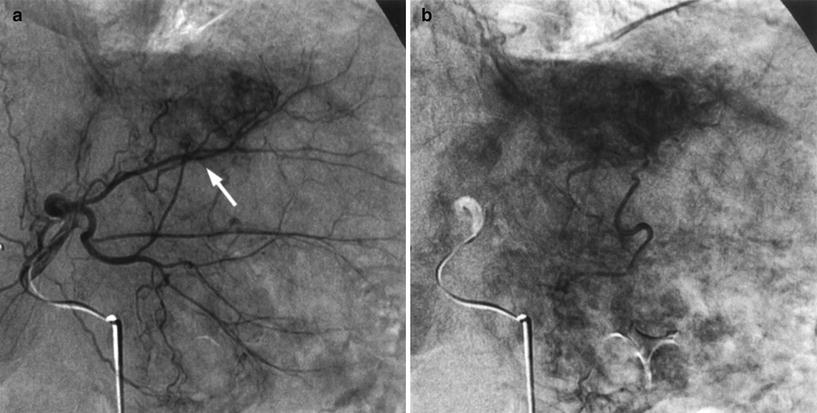

Fig. 9
a Small, yet important, accessory gastric artery (arrow) coming off segment III hepatic artery only seen after superselective catheterization. b Existence of venous gastric stain confirms the presence of the artery
3.1 Vessels Originating from the CHA and Proper Hepatic Artery
3.1.1 Gastroduodenal Artery and Pancreaticoduodenal Arcade
The GDA is usually not only the largest extrahepatic vessel but also one that is almost constantly present, having many possible origins, the common hepatic artery (92.3 %) being the most common. Together with the pancreaticoduodenal arcade it supplies blood to the duodenum, pancreas, and stomach and forms an important part of the anastomotic system between the celiac axis and the superior mesenteric artery, having the potential to become important collaterals in cases of celiac stenosis, providing a potential route for redirection of flow (Liu et al. 2005; Song et al. 2002) (Fig. 10). Because of this it is of utmost importance to carry out a proper angiographic assessment and consider prophylactic occlusion of this vessel prior to radioembolization in order to avoid pancreatitis or duodenal ulceration and perforation (Carr et al. 1997).
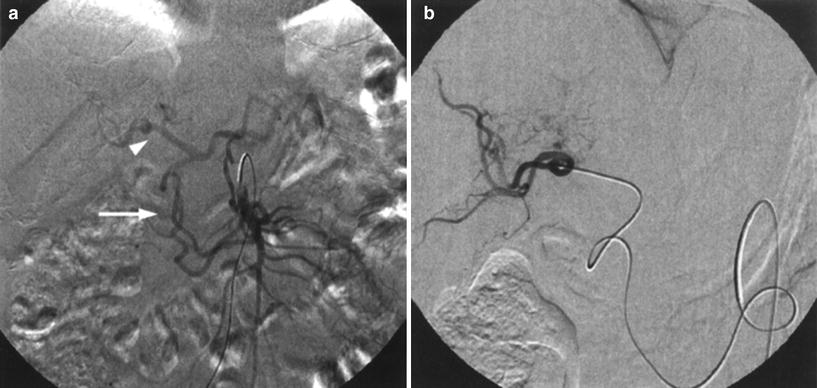

Fig. 10
Patient with a stenosis of the celiac trunk. a Superior mesenteric angiography demonstrates reversed flow through the pancreaticoduodenal arcade (arrow) with opacification of the hepatic artery (arrowhead). b Tumor was treated after selective catheterization of the right hepatic artery through the collateral network
The pancreaticoduodenal arcade provides an extensive collateral vascular network to the head of the pancreas, uncinate process, and duodenal bulb with a complex anatomical disposition and anastomotic channels with named arteries such as the dorsal pancreatic artery, the supraduodenal artery, and the retroduodenal artery (Hentati et al. 1999; Bertelli et al. 1995, 1996a, b, 1997, 1998).
The retroduodenal artery (Michels 1951) or posterior pancreaticoduodenal arcade arises as the first branch of the gastroduodenal artery crossing anterior to the supraduodenal portion of the common bile duct, and then behind the intrapancreatic portion of the common duct to form an arterial arcade on the posterior surface of the head of the pancreas with numerous branches to the duodenum (Figs. 11 and 12). This artery gives rise to multiple arteriolar branches that contribute the blood supply to the common hepatic duct (Arias Fernández et al. 2011). It has been reported that the inflow of the retroportal artery in the right hepatic lobe may result in a poor distribution of the radioembolization microspheres, whereas its selective embolization resulted in an improvement of the microspheres distribution (Yamagami et al. 2005). Its catheterization may be easier from the SMA (Arias Fernández et al. 2011).
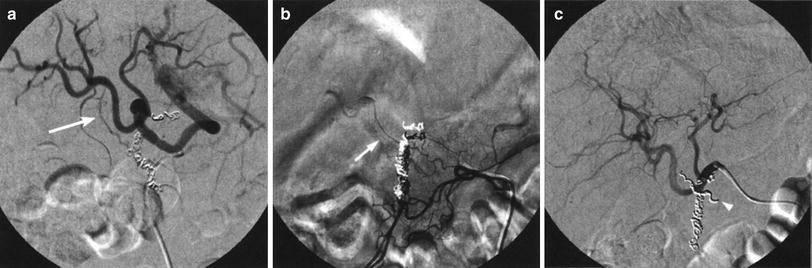
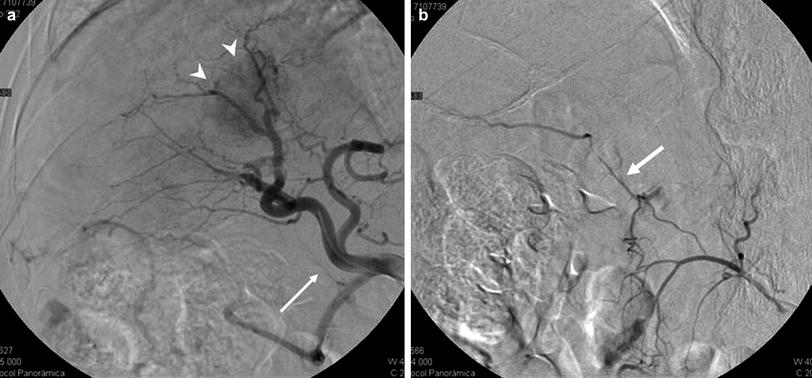

Fig. 11
Angiography performed after coiling of the gastroduodenal and right gastric arteries. a An oblique view shows the presence of an extrahepatic artery (arrow) that arises from the right hepatic artery. b Selective catheterization of the retroduodenal artery (arrow) which originates in the inferior pancreaticoduodenal arcade. c Angiography performed after occlusion of the retroduodenal artery (arrowhead)

Fig. 12
a Hepatocellular carcinoma in the right lobe (arrowheads). Arteriography from the CHA. Opacification of the retroportal artery (arrow). b Contrast injection from the SMA. Retrograde filling of the retroportal artery (arrow) with contrast passing into the branch of segment VI (with permission from: Arias Fernández et al. 2011)
The dorsal pancreatic artery is the first major pancreatic branch, usually coming off the splenic artery, although many variations have been described (right hepatic artery, SMA, and celiac artery) (Arias Fernández et al. 2011). After supplying the dorsal surface of the neck of the pancreas, it divides into a left branch, the transverse pancreatic, and into a right branch (branches), which unites with the gastroduodenal or the superior pancreaticoduodenal (Michels 1951).
The transverse pancreatic artery is one of the major arteries of the pancreas and generally the major left branch of the dorsal pancreatic. It courses along the inferior surface of the pancreas to unite with the a. pancreatica magna (branch of the splenic artery) (Michels 1951).
The supraduodenal artery (of Wilkie) (Michels 1951) is a small artery that supplies blood to the anterior and posterior surfaces and upper portion of the duodenum and pylorus, and communicates with the pancreaticoduodenal arcade, as well as right gastric branches. It is reported to be present in 93 % of patients, with a high degree of variation in its origin, the most frequent being the gastroduodenal artery (27 %), followed by the common hepatic artery (20 %), left hepatic artery (20 %), right hepatic artery (13 %), and cystic arteries (10 %). Duplication of the artery has been described in 2–3 % of cases (Van Damme and Bonte 1990; Bianchi and Albanese 1989).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree