Fig. 1
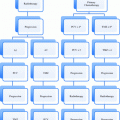
Preoperative chemoradiation for rectal cancer: decisions based on clinical staging (* Local clinical staging based on exam, EUS and MRI; distant disease staging based on full body PET/CT or CT of chest, abdomen and pelvis. # Select patients with low-lying distal cT2 lesions may be considered for preoperative chemoradiation in attempts to convert an APR to a sphincter sparing TME ^ Chemotherapy concurrent with long-course radiation may be 5-FU or capecitabine). Of note, all clinical stage II and III patients receive additional postoperative chemotherapy
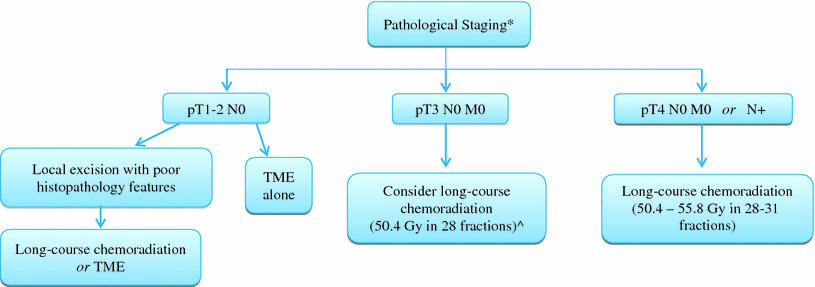
Fig. 2
Postoperative chemoradiation for rectal cancer: decisions based on pathologic staging (* No preoperative chemoradiation was administered ^ If high lesion with negative margins and good histopathology features, may consider omitting chemoradiation). Of note, may consider adjuvant chemoradiation for any margin positive status, and all stage II and III patients receive additional postoperative chemotherapy
For clinical stage II–III rectal cancers (T3-T4N0 or N +) implying locally advanced disease, preoperative treatment is indicated. The German Rectal Cancer Study Group CAO/ARO/AIO-94 phase III trial compared preoperative long-course radiotherapy (50.4 Gy in 28 fractions) followed by TME, with TME followed by postoperative standard chemoradiation (54 Gy over 6 weeks with concurrent 5-FU). Patients in the preoperative radiation arm had a decreased risk of local recurrence at 10 years (7.1 vs. 10 %, p = 0.048), improved acute and late morbidity, enhanced tumor downstaging, and an increased rate of sphincter-preserving surgery for those declared upfront to have required an APR, relative to the postoperative chemoradiation arm (Sauer et al. 2004, 2012). There was, however, no difference in overall or disease-free survival between preoperative and postoperative chemoradiation. Of note, both arms received 4 cycles of chemotherapy outback, and the rate of distant failures was the same in both of these groups (29.8 and 29.6 % at 10 years, p = 0.9) (Sauer et al. 2012).
In the United States, preoperative treatment for stage II–III cancers is long-course chemoradiation to 50.4 Gy with concurrent 5-FU-based chemotherapy (continuous infusion 5-FU or oral capecitabine), rather than short-course radiation (5 Gy × 5 fractions) to 25 Gy, which is commonly used in Europe, because of the concern for heightened late effects with one week of hypofractionated radiation. These two preoperative regimens were compared head-to-head in the recently published Tasman Radiation Oncology Group (TROG) 01.04 trial; 326 patients with T3N0-2M0 ultrasound or MRI staged rectal cancer were randomized to receive either standard fractionated long-course chemoradiation followed by TME 4–8 weeks later or short-course radiation followed by TME one week later (Ngan et al. 2012). No statistical difference in rates of local or distant recurrence or overall survival was reported at a relatively short follow-up of 3 years. However, in a subset analysis of patients with distal (<5 cm from the anal verge) tumors, there was an observed difference in local recurrence rate favoring long-course preoperative chemoradiation (12.5 % in the short-course arm compared with 0 % in the long-course arm, p = 0.21). Pathologic downstaging was also significantly more common in the long-course preoperative arm (45 vs. 28 %), though the rate of APR was similar (79 and 77 %). An increased risk of local recurrence was associated with older patients, poorer ECOG performance status, positive lymph nodes, and increased CEA level. Notable, 56 % of patients in both arms had T3N0 disease, which may have led to the similar outcomes, and another criticism was the different duration of treatment in the two arms (patients in the short-course radiation arm go through approximately 1 week of preoperative treatment before surgery versus the 2–4 months before surgery in the long-course arm). It is unclear whether this difference in timing affected outcome, and this is anticipated to be one of the analyses performed for the ongoing Stockholm III three arm randomized trial comparing short-course radiation followed by TME one week later, with short-course radiation or long course–chemoradiation followed by TME 4–8 weeks later (Pettersson et al. 2010).
One clinical scenario potentially favoring preoperative short-course radiation is oligometastatic disease. In a phase II study by the Dutch Colorectal Group, 50 patients with primary rectal cancer presenting with synchronous resectable metastasis received short-course preoperative pelvic radiation followed by six cycles of capecitabine, oxaliplatin and bevacizumab, and then resection of the primary as well as resection and/or ablation of the metastasis (van Dijk et al. 2010). Of the 41 patients who proceeded to surgery, 44 % achieved a pCR or near-pCR of the rectal tumor. No notable toxicities were observed during radiation or peri-operatively, and no progression was noted during chemotherapy. Another phase II trial for patients with cT3-4, any N and any M rectal cancer examined preoperative short course radiation followed by 4 cycles of mFOLFOX6 chemotherapy prior to surgery (Myerson et al. 2012). Of the 44 evaluable cases, 33 had ypT0-2 residual disease. These two studies suggest that short-course preoperative radiation may be a reasonable strategy to achieve durable disease-free survival and local palliation in patients with low burden metastatic disease. Some institutions also consider neoadjuvant short-course radiation a valid alternative in patients with cT3N0 rectal cancer whose disease does not need downsizing (not threatened by a close circumferential margin; located in the upper and mid rectum) (Mohiuddin et al. 2008). Adjuvant chemotherapy decisions are then made based on pathological data.
In the U.S., concurrent adjuvant postoperative chemotherapy is administered for patients with clinical stage II or III cancer that received preoperative chemoradiation or for those that underwent upfront TME and had pathologic stage II or III disease receiving chemoradiation postoperatively. The efficacy of postoperative radiation therapy and 5-FU-based chemotherapy for stage II and III rectal cancer was established by a series of prospective, randomized clinical trials from the Gastrointestinal Tumor Study Group (GITSG-7175), the Mayo/North Central Cancer Treatment Group (NCCTG-794751), and the National Surgical Adjuvant Breast and Bowel Project (NSABP-R-01) (Thomas and Lindblad 1988; Krook et al. 1991; Wolmark et al. 1988). These studies demonstrated an increase in both disease-free and overall survival when postoperative chemoradiation and four additional cycles of adjuvant 5-FU were administered.
While chemotherapy with fluoropyramidines has shown to enhance local control and the pCR rate when administered concurrently with long course pelvic radiation, the addition of 5-FU based chemotherapy after surgery has not conferred a survival benefit following preoperative chemoradiation (Bosset et al. 2006). The European Organization for Research and Treatment of Cancers (EORTC) 22921 study showed that there was no significant impact on overall survival with either pre- or postoperative chemotherapy (HR for death in the preoperative chemoradiation group was 1.02 compared with the preoperative radiation group; and the 5-year overall survival rate was 63.2 % for patients that did not receive adjuvant chemotherapy as compared to 67.2 % for patients receiving 5-FU postoperatively). However, an unplanned subset analysis of patients who underwent complete resection with negative margins and had M0 disease at surgery showed that ypT0-2 patients appeared to experience a survival benefit from adjuvant 5-FU as compared with ypT3-4 patients; patients in whom no downstaging was achieved did not benefit (Collette et al. 2007). Despite these findings, many academic oncologists recommend that 5-FU and more recently FOLFOX be considered as adjuvant chemotherapy in rectal cancer. However, there are no data in preoperatively treated clinical stage II or III rectal cancer to support this consideration.
3 Prognostic Factors
The prognosis of patients with localized rectal cancer is associated with several factors including the depth of tumor penetration through the bowel wall, involvement of pelvic lymph nodes, positive circumferential (radial) margin, and response to preoperative chemoradiation. However, only disease stage and circumferential margin status has been validated in multi-institutional prospective studies (Table 1).
Table 1
Predictors of local recurrence in rectal cancer
Prognostic factor |
|---|
Clinical |
T stage |
Node-positive |
Pathologic |
Circumferential margins <2 mm |
Treatment response to preoperative chemoradiation |
3.1 Disease Stage (Tumor and Nodal)
The American Joint Committee on Cancer (AJCC) staging system is universally recommended and describes the anatomical extension of rectal tumors (American Joint Committee on Cancer 2010), Table 2. Its prognostic value is based on data derived from the outcome of patients after complete surgical resection with or without combined modality therapy. Both tumor penetration of the bowel wall (the T stage) and lymph node involvement (the N stage) are associated with an increased risk of local recurrence, distant relapse and overall survival (Gunderson et al. 2002, 2004). In pooled analyses of 2551 patients from three phase III North American postoperative localized rectal cancer trials, both overall and disease-free survival were dependent on TN stage, and treatment method (Gunderson et al. 2002). Three patient risk groups were defined: intermediate- (T1-2N1, T3N0), moderately high- (T1-2N2, T3N1, T4N0), and high- (T3N2, T4N1, T4N2) risk. Patients with a single high-risk factor (T1-2N1, T3N0) had better overall survival, disease-free survival, and local disease control than patients with both high-risk factors; surgery and chemotherapy (without radiation) for these patients resulted in a 5-year overall survival rate of approximately 85 %.
Table 2
AJCC TNM classification of rectal cancer (clinical or pathological)
Stage Description | |
|---|---|
Primary tumor (T)a | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ, intraepithelial or invasion of the lamina propria |
T1 | Tumor invades submucosa |
T2 | Tumor invades muscularis propria |
T3 | Tumor invades through the muscularis propria into perirectal tissue |
T4a | Tumor penetrates to the surface of the visceral peritoneum |
T4b | Tumor directly invades or is adherent to other organs or structures |
Regional lymph nodes (N)b | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1a | Metastasis in 1 node |
N1b | Metastasis in 2–3 regional nodes |
N1c | Tumor deposits in the subserosa, mesentery, or nonperitonealized perirectal tissues without regional nodal metastasis |
N2a | Metastasis in 4–6 regional nodes |
N2b | Metastasis in 7 or more regional nodes |
Distant metastasis (M) | |
MX | Distant metastasis cannot be assessed |
M0 | No distant metastasis |
M1a | Metastasis confined to one organ site (liver, lung, ovary, nonregional lymph node) |
M1b | Metastases in more than one organ/site or the peritoneum |
The Intergroup 0114 adjuvant trial for rectal cancer, comparing the addition of leucovorin and/or levamisole to 5-FU chemotherapy, demonstrated that even in the TME era, adjuvant therapy was beneficial in patients with resectable T3-4 or N1-3 disease, with no distant metastases (Tepper et al. 2002). Within this locally advanced group of patients, those with T4 or T3N+ disease had significantly lower overall survival (55 % at 5 years) and disease-free survival (44 %), compared to patients with T1-2N+ or T3N0 disease who had a 5-year overall survival of 76 % (RR 2.1, p < 0.0001) and disease-free survival of 67 % (RR 2.0, p < 0.0001). Local recurrence also varied by stage: the higher risk group of T4 or T3N+ disease had double the local failure rate at 5 years than did the T1-2N+ or T3N0 patients (18 vs. 9 %, respectively, p < 0.0001). Interestingly, this prospective study also found that males had a worse overall survival than females (RR 1.2, p = 0.03), though disease-free survival or local recurrence was not significantly different by gender. There was no interaction between gender and stage, although it was found that females experienced more toxicity than males (81 % females reported grade 3–5 toxicity compared with 69 % of males).
3.2 Surgical Margins
Another important prognostic factor is the circumferential or radial surgical margin status. The circumferential margin is a surgically created plane of dissection produced during the removal of the mesorectum and rectum from its surroundings during a TME. Retrospective studies have confirmed a strong association between the presence of microscopic tumor cells within 1 mm of the circumferential margin and increased risks of both local recurrence and disease-free survival, even with meticulous TME surgery (Wibe et al. 2002). Macroscopic or microscopic margin radial positivity was recently shown to be a significant risk factor for local recurrence, resulting in a hazard ratio of 6.46 (p < 0.001) in the randomized TROG study previously described (Ngan et al. 2012).
3.3 Tumor Downstaging Following Preoperative Chemoradiation
The degree of tumor regression following preoperative chemoradiation has also been demonstrated to be prognostic for outcome. In the experience of the German Rectal Cancer Study Group, using a histology grading system that analyzes cytologic alterations in response to neoadjuvant treatment, patients whose tumors have no evidence of viable tumor cells in the rectal wall (tumor regression grade 4) had improved disease-free survival (86 % at 5 years) and metastases-free survival, compared with patients whose tumors showed no regressive changes post-treatment (Rödel et al. 2005). Patients with tumors showing intermediate tumor regression yielded an intermediate prognosis (disease-free survival 75 % at 5 years), and poor tumor regression predicted for an unfavorable outcome (disease-free survival 63 % at 5 years). As all stage II and III rectal cancers are recommended to receive adjuvant chemotherapy following resection, tumor downstaging is currently not used to determine chemotherapy-based management decisions.
3.4 Molecular Markers
Preoperative chemoradiation for localized rectal cancer also provides investigators with the opportunity to identify predictive and prognostic markers for treatment response. This may lead to the potential for individualizing treatment regimens. Although there are many biomarkers that have been evaluated in this regard, none have been validated for clinical use. This is an area that warrants continued research. In terms of response to systemic therapy, some molecular markers that are known to confer a poorer prognosis include microsatellite instability, 18q loss of homozygosity, epidermal growth factor overexpression, KRAS mutations, and the BRAF V600E mutation (Tol et al. 2009; Di Nicolantonio et al. 2008; Fallik et al. 2003; Des Guetz et al. 2009; Khambata-Ford et al. 2007).
4 Investigations into Selective Treatment
4.1 Omission of Pelvic Radiation
While several clinical trials have established that trimodality therapy (surgery with chemoradiation) improves local recurrence rates over surgery alone, there is ongoing investigation to identify patients who are at low-risk for pelvic recurrence, in whom preoperative radiation may be omitted. In a retrospective review of patients with pathologic stage T3N0 rectal cancer who underwent resection without pelvic radiation or chemotherapy, patients with favorable histologic features (well- or moderately well-differentiated carcinomas invading <2 mm into perirectal fat, without lymphatic or venous vessel involvement) experienced a local control and recurrence-free survival advantage (95 and 87 %, respectively), relative to patients whose tumors exhibited deep perirectal fat invasion, vessel involvement, or poor differentiation (71 and 55 %, respectively) (Willett et al. 1999).
In Europe, rectal MRI findings (the extramural extent of the tumor, the relation of the tumor to the mesorectal fascia, and the presence of suspicious lymph nodes) have been used to stratify patients into low-, intermediate-, and high-risk groups for preoperative radiation management decisions. Patients with MRI determined low-risk disease are treated with surgery alone, while those with intermediate-risk rectal tumors receive short course radiotherapy, and patients with high-risk tumors are administered long course preoperative chemoradiation (Smith and Brown 2008).
Perhaps pelvic radiation may be omitted in patients who have a good response to induction chemotherapy. A Phase II study at Memorial Sloan Kettering Cancer Center assessed 6 cycles of preoperative FOLFOX with bevacizumab in 30 patients with stage II-T3N0-any rectal cancer (Schrag et al. 2010). At preoperative restaging with sigmoidoscopy and EUS, all patients were deemed to have a complete response and underwent TME without pelvic radiation. An R0 resection (complete resection with negative margins) was performed in all patients, and the pCR rate was 27 %. Follow-up thus far is limited; so information on pelvic control and survival are not yet available. However, based on this encouraging pilot data, the PROSPECT trial, a large U.S. phase II/III randomized trial, is actively accruing patients to determine whether those with locally advanced rectal cancer undergoing low anterior resection with TME may be treated with preoperative chemotherapy and selective, rather than routine, use of pelvic radiotherapy prior to resection. Pelvic radiation will be omitted in those patients having a good response to FOLFOX therapy as determined by imaging studies (N1048, Schrag 2012).
4.2 Wait-and-See Approach for Surgery
Preoperative chemoradiation may lead to pCR in approximately 20 % of patients. As such, the option of nonoperative therapy is being evaluated in patients who have a clinical complete response to neoadjuvant therapy. The feasibility of this approach was initially reported by investigators from Brazil, who observed 99 patients with a clinical complete response to preoperative chemoradiation without radical resection (Habr-Gama et al. 2006). The local recurrence rate was remarkably low (5 %), and all recurrences have been salvaged. This “wait-and-see” policy has also been studied prospectively by a group from the Netherlands in 21 patients who achieved clinical complete response following standard chemoradiation for T4 or T3N1-2 rectal cancer, based on MRI, endoscopy, digital rectal exam, and biopsies (Maas et al. 2011). Patients with node-positive disease at initial staging (16 of the 21 patients) had adjuvant chemotherapy (capecitabine and oxaliplatin). With a median follow-up of 15 months, only one patient in the wait-and-see group developed a local recurrence at 22 months of follow-up that was successfully salvaged. All other 20 of the patients were alive and disease-free, with better bowel function than a control group of patients who had pathologic complete response at surgery. Overall survival and disease-free survival were comparable in the wait-and-see and control groups, suggesting that surgery may be avoided in a very carefully selected group of patients following chemoradiation.
5 Toxicity
5.1 Acute and Late Morbidity from Long-Course Chemoradiation
Pelvic chemoradiation can be associated with both acute and long-term toxicities due to the radiosensitivity of the surrounding normal structures including bowel, bladder, genitalia and bone. The German Rectal Cancer Study demonstrated that both physician-reported acute and late toxicity was improved with a preoperative chemoradiation approach (Sauer et al. 2004). Grade 3 or 4 acute toxic effects occurred in 27 % of the patients in the preoperative treatment group, as compared with 40 % of the patients in the postoperative treatment group (p = 0.001); the corresponding rates of long-term toxic effects were 14 and 24 %, respectively (p = 0.01). They have not yet determined any predictors for radiation-related morbidity.
The most common toxicity of pelvic chemoradiation is gastrointestinal. Acute symptoms resulting from adverse effects of the gastrointestinal tract include gas, diarrhea, rectal emptying problems, frequent bowel movements and incontinence, with late effects of obstruction due to stenosis or adhesions and more rarely malabsorption, necrosis, perforation, and fistulation (Coia et al. 1995; Sauer et al. 2004). Physician-reported grade 3 and higher diarrhea was demonstrated in 36 % of patients in the preoperative chemoradiation arm of the NSABP R-03 trial, while Bosset and colleagues reported ≥ grade 2 diarrhea in 38 % of patients treated with preoperative 5-FU and pelvic radiation (Roh et al. 2009; Bosset et al. 2006). The incidence of small bowel obstruction requiring surgery following adjuvant pelvic radiation for rectal cancer is 4–15 % in historical series (Collette et al. 2007), with a risk of late anastomotic strictures of 4–12 % (Sauer et al. 2004). Investigations have attempted to determine predictors of physician-reported small bowel toxicity. The primary predictor is increasing radiation dose. Dose-volume relationships between the amount of small bowel receiving low- and intermediate-doses of radiation and the rates of severe diarrhea have been demonstrated, but not validated in prospective multi-institutional studies (Tho et al. 2006; Baglan et al. 2002).
Interestingly, it has also been demonstrated that gastrointestinal toxicity rates may be interpreted differently by patients. Seventy-seven consecutive patients receiving preoperative chemoradiation at Harvard radiation oncology departments completed a 7-item Bowel Problems Scale immediately before weekly physician visits (Chen et al. 2010). By week 5 of long-course chemoradiation, approximately 40 % of all patients developed clinically meaningful pain, bowel urgency, or tenesmus that was not present during week 1; 30 % developed diarrhea, abdominal cramping, and passing mucus. Within each physician-assessed grade of diarrhea, patient experience varied widely. The same group also evaluated dose-volume predictors of gastrointestinal toxicity from the patient’s perspective in this patient cohort (Chen et al. 2012). The amount of small bowel receiving at least 15 Gy was significantly associated with acute symptoms (p = 0.01). These studies highlight the importance of including patient-reported morbidity and associated dose-volume predictors in prospective trials.
Urogenital dysfunction after chemoradiation for rectal cancer is also common, but to a much lesser degree than gastrointestinal toxicity. Acute morbidity may include incontinence, retention, dysuria, frequency and urgency. Late urinary tract symptoms have been reported in 2–4 % of all patients in the German study (Sauer et al. 2004).
Pelvic radiation for rectal cancer may also lead to increased sexual dysfunction, although rates of this toxicity are not well defined. In males, a long-term deterioration of ejaculatory and erectile function is due to late radiation damage to the seminal vesicles and small vessels, respectively. In females, radiation leads to vaginal dryness as well as varying degrees of vaginal fibrosis, with a resultant diminished sexual satisfaction (Marijnen et al. 2005). Dose-volume predictors for sexual dysfunction have not been reported. In females, vaginal dilators following completion of pelvic radiation are often recommended, although their efficacy has not been confirmed in randomized trial data.
Additionally, femoral head and pelvic fractures are a late complication of pelvic radiation that appears to be significantly increased in irradiated patients (Bruheim et al. 2010). In the Norwegian study by Bruheim et al. the incidence of pelvic fracture was five times higher in the irradiated patients (5 vs. 1 %) and suggested to be dose-related (Bruheim et al. 2010). However, female sex appears to be the only independent predictor for fracture likely due to age and hormone-related bone loss (Baxter et al. 2005).
5.2 Role of Conformal Radiotherapy
While there is an advantage of neoadjuvant chemoradiation followed by TME over postoperative adjuvant therapy in terms of tolerability and local control, acute gastrointestinal toxicity remains a limiting factor (Sauer et al. 2004). For example, 36 % of patients in the preoperative arm of the NSABP R-03 trial experienced ≥ grade 3 diarrhea, while Bosset et al. reported ≥ grade 2 diarrhea in 38 % of patients treated with preoperative 5-fluorouracil and pelvic radiation (Roh et al. 2009; Bosset et al. 2006).
These rates of acute gastrointestinal toxicity are due, in part, to the large amount of normal small bowel that is in the standard pelvic radiation field. Dose-volume relationships between the amount of small bowel receiving low- and intermediate-doses of radiation and the rates of severe diarrhea have been demonstrated (Tho et al. 2006; Baglan et al. 2002). Finding strategies to reduce acute gastrointestinal toxicity may potentially lead to unplanned chemoradiation treatment breaks, which has been shown to confer untoward local control and survival outcomes (Fietkau et al. 2007).
One technique to reduce the volume of irradiated small bowel is the use of prone positioning with a bowel displacement device (belly-board) (Gunderson et al. 1985). More recently, there has been investigation in the use of highly conformal treatment approaches, such as intensity modulated radiation therapy (IMRT). Compared to conventional two- or three-dimensional conformal radiation therapy (3D-CRT) planning methods, IMRT allows discriminatory dose escalation to the target volume while minimizing radiation exposure to adjacent normal tissues. Improvements in treatment-related morbidity have been described in patients treated with IMRT for other pelvic malignancies including anal, gynecologic and prostate (Kachnic et al. 2012a, b, c; Ashman et al. 2005; Mundt et al. 2002).
To this end, we performed a retrospective analysis of 48 consecutive patients treated with preoperative chemoradiation for rectal cancer to a planned dose of 45–50 Gy using 3D-CRT or IMRT while prone on a bowel displacement device (Parekh et al. 2010). There was a significant reduction in grade ≥2 gastrointestinal toxicity (NCI-CTC v3) between IMRT (30 %) and 3D-CRT (60.7 %), (p = 0.036), and grade ≥2 diarrhea: IMRT (10 %) and 3D-CRT (42.8 %), (p = 0.014). Radiation duration was significantly less with IMRT, 35 versus 39 days using 3D-CRT, (p = <0.0001). While pCR rates were 16.7 % for 3D-CRT and 21.4 % for IMRT, when also including patients with only microscopic residual disease, pCR plus microscopic rates were 57.1 % for IMRT and 27.8 % for 3D-CRT, (p = 0.093). These results are consistent with the published observations from the Mayo Clinic, which showed a similar reduction in grade 2+ gastrointestinal toxicity with the use of IMRT (Samuelian et al. 2011).
The Radiation Therapy Oncology Group (RTOG) recently completed the phase II 0822 trial examining the role of preoperative IMRT in combination with capecitabine and oxaliplatin. Preliminary results presented only in abstract form have suggested a small, but insignificant benefit in GI toxicity with IMRT as compared to patients treated with 3D-CRT on the RTOG 0247 trial (Garofalo et al. 2011). Although these data require further analysis, they may be due to the lack of maximal bowel displacement and heterogeneous method of contouring small bowel. Despite these early results, the optimization and further analysis of this IMRT approach in the combined modality management of locally advanced rectal cancer is warranted.
6 Anal Cancer Introduction
Anal squamous cell cancer is a rare malignancy and accounts for only 4 % of all cancers of the lower gastrointestinal tract. The estimated new cases from anal cancer in the United States in 2013 are 7,060 resulting in approximately 880 deaths (American Cancer Society 2013 Facts and Figures). In the last decade, the incidence of anal cancer is increasing due to the association with human papilloma virus (HPV) infection (Johnson et al. 2004). The incidence of anal cancer is also higher in those who are Human Immunodeficiency Virus (HIV) positive due to immunosuppression, and in transplant recipients (Johnson et al. 2004). In contrast to other gastrointestinal tract malignancies, anal cancers are highly sensitive to chemoradiation alone. Preservation of anorectal function occurs in the majority of cases, preserving APR for local–regional recurrences.
The anal canal begins at the narrowing of the rectal ampulla at the anorectal junction where the rectum enters the puborectalis sling at the apex of the anal sphincter complex, and extends distally for approximately 3–4 cm and ends at the anal verge. This is in distinction from an anal margin or skin cancer.
7 General Treatment Paradigm
Whereas surgical management with a permanent colostomy was the first-line approach to treating anal canal cancer prior to the 1980s, organ preservation with chemoradiation is now the mainstay of treatment due to the contributions of Dr. Norman Nigro from Wayne State University (Nigro et al. 1977). Dr. Nigro initially evaluated chemoradiation, 30 Gy concurrent with 5-FU and mitomycin-C (MMC) chemotherapy, as neoadjuvant therapy prior to an APR in an effort to improve pelvic control. When it was shown that pathologic complete responses were induced in 24 of 28 patients, surgery was then reserved for salvage of local–regional persistent or recurrent cancer.
Four randomized clinical trials have since validated chemoradiation with both 5-FU and MMC as the standard treatment of anal canal cancer (Northover et al. 2010; Bartelink et al. 1997; Flam et al. 1996; Ajani et al. 2008). The United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Anal Cancer Trial (ACT I) and the European Organization for Research and Treatment of Cancer (EORTC) phase III studies had similar designs, comparing 5-FU, MMC and radiation to radiation alone, but employed higher radiation doses (45 Gy plus a boost) than the original Nigro trial (Northover et al. 2010; Bartelink et al. 1997). Both studies demonstrated improved local–regional control with the addition of doublet chemotherapy over radiation alone, which translated into higher cancer-specific survival in the ACT I trial and improved colostomy-free survival in the EORTC investigation. There was no statistically significant survival difference in either study between the two arms, likely in part due to the ability of APR to salvage persistent or recurrent local–regional disease.
Further investigative attempts focusing on reducing the acute toxicity, particularly myelosuppression of chemoradiation, by removing or replacing the MMC from the Nigro regimen, have been unsuccessful. Elimination of MMC in the RTOG 87-04 phase III trial, which randomized patients to radiation (45–54 Gy) and 5-FU (delivered as continuous infusion in week 1) with or without MMC (delivered weeks 1 and 5) resulted in an almost doubling of 5-year local recurrence, an increase in colostomy rate (23 vs. 9 % at 4 years, p = 0.002), and a decrease in 5 year disease-free survival (from 64 % in the 5-FU/MMC arm to 50 % in the 5-FU alone arm, p < 0.003) (Flam et al. 1996). Moreover, the addition of MMC to 5-FU and radiation appeared to be most beneficial to patients with T3-4 or N0 disease, in terms of colostomy rate reduction.
More recently, cisplatin has been studied as a substitute for MMC in the RTOG 98-11 trial. Six hundred and eighty-two patients with stage T2N0 and above non-metastatic anal canal cancer were randomized to receive either radiation with 5-FU/MMC (per the RTOG 87-04 study), or to receive induction 5-FU/cisplatin for 2 cycles followed by radiation with 5-FU/cisplatin. Radiation was in general a two-dimensional delivery approach with 45 Gy to the pelvis followed by a boost to gross disease at the discretion of the physician to up to 59.4 Gy. Although there was no statistically significant difference in 3-year overall or disease-free survival between the two groups, local–regional relapse rates were higher in the cisplatin-treated patients (33 vs. 25 %; p = 0.07), while colostomy-free survival was significantly lower (90 vs. 81 %; p = 0.02) (Ajani et al. 2008). In the most recent update, the use of cisplatin was now shown to be inferior to MMC in terms of both overall survival and disease-free survival (5-year disease-free survival: 67.8 vs. 57.8 %, p = 0.006; 5-year overall survival: 78.3 vs. 70.7 %, p = 0.026), (Gunderson et al. 2012). One potential limitation of the RTOG 98–11 trial was the use of induction chemotherapy, which may have accounted for the poorer outcomes as it delayed the initiation of definitive chemoradiation. To this end, neither induction or outback 5-FU and cisplatin chemotherapy, nor higher radiation doses, have been proven effective in enhancing outcomes in anal canal cancer (Peiffert et al. 2012; James et al. 2009).
To guide chemoradiation treatment decisions in anal canal cancer, preoperative staging, which is based on gross tumor volume and nodal involvement, is critical. As such, anal canal cancers are staged by physical examination and imaging studies. Digital rectal examination can determine the sphincter tone, size (which is a main indicator for AJCC T staging), location, and degree of fixation to adjacent structures of the primary tumor, while palpation of the groins can indicate nodal enlargement (AJCC N staging), (American Joint Committee on Cancer 2010), Table 3. Female patients should undergo a gynecological examination to exclude other HPV-associated cancers. Sigmoidoscopy is also warranted as it provides additional information about the primary and its mucosal spread. Exam under anesthesia is sometimes necessary due to pain upon the digital rectal evaluation. Determination of HIV status is also important in guiding clinical treatment decisions and will be discussed in Sect. 3.
Table 3
Predictors of local recurrence in anal canal cancer
Adverse prognostic factor |
|---|
Clinical |
Lymph node involvement |
Tumor diameter >5 cm |
Male sex |
Treatment breaks during chemoradiation |