Fig. 1
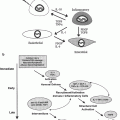
After completion of therapy clinical summary for an adult survivor of childhood acute lymphoblastic leukemia detailing therapeutic exposures and health screening recommended by the Children’s Oncology Group
It is anticipated that early detection of health problems, institution of preventive or remedial therapy, and modification of maladaptive health behaviors will provide aging childhood cancer survivors with opportunities to maintain or improve health. Implementation of risk-based care requires a working knowledge about health risks predisposed by treatment for childhood cancer, an expertise that is likely to be available only at a long-term follow-up program in a pediatric cancer center (Aziz et al. 2006). An essential service of these multidisciplinary programs is to facilitate the transition from oncology to community care by organizing an individualized survivorship care plan that includes details about therapeutic interventions undertaken for childhood cancer and their potential health risks, personalized health screening recommendations, and information about lifestyle factors that modify risks. The survivorship prescription is extremely important because the survivor’s contact with the cancer center becomes less frequent with increasing passage of time from diagnosis and therapy (Oeffinger et al. 2004).
Coordination of risk-based care becomes more challenging as survivors age out of pediatric long-term follow-up programs and return to community providers for both their primary and cancer-related care. Research has demonstrated that community providers are largely unfamiliar with the health risks associated with childhood cancer, which includes a heterogeneous group of relatively rare diseases managed with diverse therapeutic approaches that have evolved over the years (Landier et al. 2006). Access to preventive care may also be hindered by survivors who are uninformed about their cancer history and its associated health risks (Kadan-Lottick et al. 2002).
Because most survivors do not have access to late effects experts in their community to coordinate health care after cancer, patient education and self-advocacy have been promoted as a means of disseminating awareness about cancer-related health risks to community providers (Landier et al. 2006). Resources that provide busy clinicians with accurate and succinct information about cancer-related late effects can help survivors gain access to appropriate health screening. To address this need, several groups have developed guidelines aiming to facilitate and standardize risk-based care of childhood cancer survivors (Skinner et al. 2005). Limitations in high quality health outcomes investigations posed challenges in efforts to organize an evidence-base to support specific screening recommendations. Deficiencies encountered included the lack of standard definitions of toxicity, use of variable testing strategies, and inconsistency in evaluation time in relation to therapeutic exposure, and bias related to incomplete participation of at-risk cohorts. Since childhood cancer comprises a relatively small proportion of cancer diagnoses, establishing through randomized clinical trials that screening of asymptomatic survivors can reduce morbidity and mortality is not feasible. Consequently, studies evaluating utility and cost-effectiveness of screening asymptomatic survivors are unlikely to be undertaken.
Despite the considerable limitations in the evidence currently available to guide health screening recommendations for childhood cancer survivors, compelling evidence does exist linking adverse outcomes to specific therapeutic exposures. These data prompted the use of a hybrid-model for the development of health screening recommendations to address the medically vulnerable and growing population of childhood cancer survivors. Group methods have varied in the magnitude and scope of the assessment of the evidence-base of adverse outcomes. However, all proposed screening recommendations based on the collective clinical experience of late effects experts that matched the magnitude of the risk with the intensity of the screening recommendation. A brief description of the guideline development methodology and content used by the Children’s Oncology Group (COG), the Scottish Intercollegiate Guideline Network (SIGN) and the Late Effects Group of the United Kingdom Children’s Cancer Study Group (UKCCSG) is summarized in Table 1.
Table 1
Guidelines for follow-up care after childhood cancer
|
Organization
|
Search methods
|
Scoring of evidence
|
Content/Features
|
|---|---|---|---|
|
Children’s Oncology Group
Long–Term Follow–Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers
Version 1.0 released March 2003
Version 2.0 released March 2006
|
Systematic literature review via MEDLINE (National Library of Medicine, Bethesda, MD) using key search words comprised “childhood cancer therapy,” “complications,” “late effects”, and specific toxicities
|
Scoring by multidisciplinary panel of late effects experts according to a modified version of the National Comprehensive Cancer Network “Categories of Consensus” system (high to lower level of evidence)
|
136 sections of exposure-based potential late effects with periodic health screening recommendations, and supporting references.
Delineation of “At Risk” and “Highest Risk” groups
|
|
Available at: http://www.survivorshipguidelines.org
|
References from the bibliographies of selected articles were used to broaden the search”
Multidisciplinary system-based (e.g., cardiovascular, neurocognitive, reproductive, etc.) task forces monitor the literature and provide recommendations for guideline revision as new information becomes available
|
Each score reflects expert panel’s assessment of strength of evidence from the literature linking specific adverse outcomes to specific therapeutic exposures
Assessment of appropriateness of screening recommendation based on expert panel’s collective clinical experience
|
Health counseling and further considerations complemented by patient education materials handouts called “Health Links”
Adult cancer screening recommendations for standard and high-risk groups
|
|
Scottish Intercollegiate Guidelines Network
Long–Term Follow–Up of Survivors of Childhood Cancer—SIGN 76: A National Clinical Guideline
Release date January 2004
Updated March 2005
Available at: http://www.sign.ac.uk/guidelines/fulltext/76.
|
Systematic review and critical appraisal of literature using SIGN methodology
|
Rating of methodological quality (low to high risk of bias) of eligible studies by two independent reviewers.
Recommendation for evidence’s use in guiding management decisions graded (A to D) based on multidisciplinary team’s collective clinical experience and knowledge of evidence
Recommendation of “best practices” based on clinical experience of guideline development group
|
Detailed analysis of 5 key survivor outcomes:
(1) growth
(2) puberty and fertility
(3) cardiac abnormalities
(4) thyroid function
(5) neurodevelopment and psychological health
Recommendations for levels of long-term follow-up care based on intensity of treatment received
|
|
United Kingdom Children’s Cancer Study Group
Late Effects Group
Practice Statement: Therapy Based Long Term Follow Up.
1st Edition released 1995
2nd Edition released April 2005
2nd Edition updated June 2006
|
Formal literature searches of key topics
|
Assessment of data from literature review complemented by expert committee reports and opinions and clinical experience and practice of respected authorities
Formal critical appraisal undertaken in specific contributions cross-referenced to the SIGN 76 Guidelines
|
Brief summary of exposure-based outcomes by any treatment, chemotherapy, radiation, and surgery
25 key content sections highlighting potential adverse outcomes, risk factors, screening recommendations, further management, and supporting references
Appendices offering expanded detail about health outcomes in high risk groups (CNS tumors, transplant recipients), other general topics (puberty, fertility, immunization), and template of treatment summary
|
The COG and SIGN Guidelines and UKCCSG Practice Statement represent important educational resources for survivors of childhood cancer and providers who supervise their care. It is anticipated that increased awareness about adverse outcomes after childhood cancer offer the potential benefits of early identification of and intervention for late onset therapy-related complications. However, survivors may also experience potential harms including anxiety about health risks and false-positive screening evaluations. In addition, the costs of long-term follow-up care may be prohibitive for some patients. These issues are particularly relevant since the evidence supporting optimal screening methods for most outcomes and the benefits of treating asymptomatic survivors with subclinical dysfunction have not been established. Prospective and scientifically rigorous research is required to identify accurate and cost-effective screening modalities and appropriate screening frequency and to establish the impact of screening on long-term survivor health outcomes.
3 Follow-up Guidelines for Survivors of Adult-Onset Malignancies
In contrast to follow-up guidelines for survivors of pediatric malignancies, which are based on treatment exposures, guidelines for survivors of adult-onset malignancies are largely based on cancer types, in part because of the wider range of malignancies and the greater patient and clinical scenario heterogeneity in the adult population. In addition, follow-up recommendations for the adult-onset cancer survivors tend to be less comprehensive and specific, and are available only for a restricted number of primary cancer diagnosis.
One of the most well-recognized and widely-used guidelines in adult oncology is the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, developed through review of the evidence in conjunction with expert judgment by multidisciplinary panels from NCCN Member Institutions (2008). For each disease entity, guidelines for diagnosis and staging, primary intervention, adjuvant treatment, surveillance, management of recurrent, or disseminated disease, as well as guidelines for symptom management/supportive care are provided and continually updated. Table 2 summarizes the NCCN follow-up recommendations for each of the cancer sites. The algorithms for all disease entities included surveillance recommendations. However, many were intended solely for detection of relapses rather than for late effects. For several cancer sites, references were made for the follow-up and/or management of treatment-related late effects, most notably for survivors of breast cancer, head and neck cancer and Hodgkin lymphoma. For some cancer sites, the recommendations were based on anticipated adverse events related to their original cancer diagnosis rather than cancer therapy exposures. These include survivors of breast cancers who were also BRCA-1 and/or -2 carriers, survivors of patients with hereditary nonpolyposis colorectal cancer (HNPCC), survivors of melanoma or non-melanoma skin cancers whose first skin malignancy were likely related to sun exposures, and survivors of lung cancer in whom the first lung malignancy may be related to tobacco use. For these survivors the NCCN provide recommendations regarding more intensive cancer screening, prevention and health habit modifications.
The American College of Radiology Appropriateness Criteria also provide evidence-based guidelines for management of a selected number of primary cancer sites, including breast cancer, Hodgkin lymphoma, lung cancer, prostate cancer, and rectal-anal cancer (www.acr.org). The guidelines were developed by expert panels that consisted of oncologists as well as other specialists depending on the disease site. Of these disease sites, a separate topic dedicated to follow-up of patients were only available for lung cancer and Hodgkin lymphoma. For post-therapy follow-up for lung cancer, in addition to recommendations regarding follow-up for recurrences, discussions were made on the role of life-long chest computed tomography (CT) screening due to the increased risk of second lung cancer in these patients, although the frequency of the screening CT was considered controversial. For follow-up after Hodgkin lymphoma therapy, more detailed discussions were available on the follow-up of a number of late effects, including hypothyroidism, second malignancy, in particular breast cancer and lung cancer, and cardiovascular complications. Recommendations were also made regarding areas of patient counseling and education, both for raising patient awareness as well as for promoting life style changes.
Increasingly, information specific to cancer survivors are available on websites of major cancer centers as well as cancer organizations including the American Society of Clinical Oncology and the American Cancer Society (www.cancer.net; www.cancer.org). Most of these sites describe the key late effects in cancer survivors and provide additional links to other relevant sites and resources. At MD Anderson Cancer Center, follow-up guidelines, entitled “Long-Term Surveillance Guidelines Beyond 5 Years” for 15 different disease sites were listed (www.mdanderson.org). These included acute myelogenous leukemia, bladder cancer, bone cancer, breast cancer, cervical cancer, colorectal cancer, endometrial, head and neck, kidney, lung, lymphoma, melanoma, ovarian cancer, prostate cancer, and soft tissue sarcoma. Although most of these guidelines were again designed for detection of recurrence, some of the recommendations were related to the screening for second malignancy, thyroid dysfunction, and bone loss associated prior cancer therapy.
Table 2
NCCN surveillance guidelines for individual cancer types
|
Disease site
|
Surveillance guidelines
|
|---|---|
|
Acute myeloid leukemia
|
Surveillance for relapse only
|
|
Bladder cancer
|
Surveillance for relapse only
|
|
Bone cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
Survivorship prescription to schedule follow-up with multidisciplinary team
|
|
|
Extended therapy and surveillance may be necessary to address potential late effects of surgery, radiation and chemotherapy for long-term survivors
|
|
|
Breast cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
After tamoxifen, annual gynecological examination if uterus present and rapid assessment of any vaginal spotting
|
|
|
After aromatase inhibitor or ovarian failure, monitoring of bone health
|
|
|
Consider breast MRI in women at high risk of bilateral disease, e.g. BRCA-1 or -2 carriersa
|
|
|
Cardiac monitoring at baseline, 3 , 6 and 9 months after Trastuzumab therapy
|
|
|
Central nervous system cancer
|
Surveillance for relapse only
|
|
Cervical cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
Use of vaginal dilator after radiation therapy in women who wish to remain sexually active
|
|
|
Chronic myelogenous leukemia
|
Surveillance for relapse only
|
|
Colorectal cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
Surveillance post-treatment colonoscopy aimed at identifying and removing metachronous polyps given the increased risk of developing second cancersa
|
|
|
More frequent (annual) surveillance colonoscopy for patients with HNPCCa
|
|
|
Anal cancer
|
Surveillance for relapse only
|
|
Esophageal cancer
|
Surveillance/intervention for relapse
Surveillance for late effects
Some patients may require dilatation of an anastamotic or a chemoradiation-induced stricture
Nutritional counseling may be extremely valuable
|
|
Gastric cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
Vitamin B12 levels should be monitored for patients who have had proximal/total gastrectomy
|
|
|
Head and neck cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
TSH q 6–12 months if neck irradiated.
|
|
|
Speech/hearing and swallowing evaluation and rehabilitation as indicated
|
|
|
Smoking cessation counselinga
|
|
|
Dental follow-up
|
|
|
Hepatobiliary cancer
|
Surveillance for relapse only
|
|
Hodgkin lymphoma
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects after > 5 years
|
|
|
Annual blood pressure, serum glucose, lipids screening
|
|
|
Consider baseline echo/stress echo after 10 years
|
|
|
Pneumococcal revaccination q 5–7 years in patients who had splenectomy or splenic irradiation
|
|
|
Meningococcal/H. flu vaccination in selected patients
|
|
|
Consider annual influenza vaccine in high-risk patients
|
|
|
Annual TSH if neck irradiated
|
|
|
Annual chest imaging for patients at increased risk for lung cancer
|
|
|
Mammogram/Breast MRI screening 8–10 years after irradiation or by age 40
|
|
|
Counseling on reproduction, health habits, psychosocial, cardiovascular, breast self-exam, skin cancer risk
|
|
|
Kidney cancer
|
Surveillance for relapse only
|
|
Melanoma
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects
|
|
|
Patient education on skin self-exam, protection, and subsequent skin cancer risksa
|
|
|
Structured follow-up program to detect subsequent second primary melanoma and non-melanoma primary skin malignances; at least annual skin examination for lifea
|
|
|
Multiple myeloma
|
Surveillance for relapse only
|
|
Myelodysplastic syndrome
|
Surveillance for relapse only
|
|
Neuroendocrine tumor
|
Surveillance for relapse only
|
|
Non-Hodgkin’s lymphoma
|
Surveillance for relapse only
|
|
Non-melanoma skin cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects:
|
|
|
Basal cell and squamous cell
|
|
|
Complete skin examination q 6–12 months, annually for life after 3 yearsa
|
|
|
Dermatofibrosarcoma protuberans
|
|
|
Patient education on sun exposure and self examinationa
|
|
|
Merkel cell cancer
|
Prevention with oral retinoids in high-risk patients to reduce risk of subsequent skin cancersa
Aggressive treatment of precancers can prevent development of subsequent invasive tumorsa
|
|
Surveillance for relapse only
|
|
|
Surveillance for relapse only
|
|
|
Non-small cell lung cancer
|
Surveillance for relapse only
|
|
Occult primary
|
Surveillance for relapse only
|
|
Ovarian cancer
|
Surveillance for relapse only
|
|
Pancreatic adenocarcinoma
|
Surveillance for relapse only
|
|
Prostate cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects:
|
|
|
Increased risk of osteoporosis after either medical or surgical castration; recommended baseline bone density test and supplementation with calcium and vitamin D
|
|
|
If osteopenia or osteoporosis, strongly consider bisphosphonate therapy
|
|
|
Small cell lung cancer
|
Surveillance for relapse
|
|
Surveillance/intervention for late effects:
|
|
|
Smoking cessation intervention after recovery from primary therapya
|
|
|
New pulmonary nodules after 2 years follow-up should initiate work-up for potential new primarya
|
|
|
Soft tissue sarcoma
|
Surveillance for relapse only
|
|
Testicular cancer
|
Surveillance for relapse only
|
|
Thymic cancer
|
Surveillance for relapse only
|
|
Thyroid cancer
|
Surveillance for relapse only
|
|
Uterine cancer
|
Surveillance for relapse only
|
4 Specific Areas for Surveillance/Interventions
There are several well-documented late effects in long-term cancer survivors, some of which may be life-threatening, while others may have considerable impact on patients’ quality of life, that deserve special attention. Early detection at a treatable stage, and increased efforts toward risk reduction and prevention of these late effects may play an important role in improving the well-being of long-term cancer survivors.
4.1 Second Malignancy
Second malignancy is one of the most serious late effects in patients who had survived their first cancer. There is a large body of literature describing the increased risk of second malignancy among survivors of childhood cancers (Cardous-Ubbink et al. 2007; Davies 2007; MacArthur et al. 2007), as well as survivors of several adult-onset cancers, with most information available in survivors of Hodgkin lymphoma (Biti et al. 1994; Doria et al. 1995; Donaldson et al. 1999; Swerdlow et al. 2000; van Leeuwen et al. 2000; Dores et al. 2002; Ng et al. 2002; Franklin et al. 2006; Hodgson et al. 2007). Increasing data are also available on second malignancy risk after treatment for testicular cancer (Kollmannsberger et al. 1999; Travis et al. 2000, 2005), breast cancer (Boice et al. 1992; Fisher et al. 1994; Neugut et al. 1994; Roychoudhuri et al. 2004; Kirova et al. 2007), prostate cancer (Brenner et al. 2000; Pickles et al. 2002; Chrouser et al. 2005; Moon et al. 2006), cervical cancer (Boice et al. 1987; Kleinerman et al. 1995; Chaturvedi et al. 2007), and non-Hodgkin’s lymphoma (Travis et al. 1995; Mudie et al. 2006; Tward et al. 2006). The following lists a number of second malignancies that have been shown to have an increased incidence among specific cancer survivors, and in which screening and/or prevention may be warranted.
4.1.1 Breast Cancer as a Second Malignancy
An increased risk of breast cancer is clearly shown in survivors of a number of childhood malignancies and Hodgkin lymphoma, largely as a result of prior chest irradiation (Travis et al. 2003; van Leeuwen et al. 2003; Kenney et al. 2004). An excess risk of contralateral breast cancer related to radiation therapy has also been demonstrated in women treated with breast or chest wall irradiation (Boice et al. 1992; Gao et al. 2003; Hemminki et al. 2007). In addition to young age at treatment, hormonal exposures as well as other traditional breast cancer risk factors may further modify the risk (Travis et al. 2003; van Leeuwen et al. 2003). Mammography screening is a widely accepted screening modality that has been shown to significantly reduce breast cancer mortality in the general population (Frisell et al. 1997; Tabar et al. 2000; Duffy et al. 2005). Although its efficacy has not been specifically assessed in cancer survivors with breast cancer mortality as an endpoint, given the known significantly increased risk, mammography screening 8–10 years after chest irradiation is recommended in most follow-up guidelines for survivors. Breast MRI has been shown to be more sensitive than mammogram in high-risk women based on their genetic predisposition, although its role in detecting treatment-related breast cancer has not been assessed. Nevertheless, the ACS currently recommends yearly breast MRI imaging as an adjunct to mammography in women who received chest radiation between the age of 10 and 30 years (Saslow et al. 2007).
Chemoprevention with selective estrogen-receptor modulators (SERMS) have been evaluated in high-risk populations, mostly based on genetic predisposition but also including survivors of breast cancers, for breast cancer risk reduction (Cuzick 2008). Although it has not been assessed in other cancer survivors, in women at high risk due to their treatment history and other risk factors, tamoxifen for women who have completed child-bearing, and raloxifene in post-menopausal women can be considered on a case-by-case basis.
4.1.2 Lung Cancer as a Second Malignancy
An increased risk for lung cancer have been shown in survivors of Hodgkin lymphoma (Swerdlow et al. 2000; van Leeuwen et al. 2000; Dores et al. 2002; Ng et al. 2002; Travis et al. 2002; Hodgson et al. 2007), non-Hodgkin’s lymphoma (Andre et al. 2004; Mudie et al. 2006; Tward et al. 2006), breast cancer (Neugut et al. 1994; Kaufman et al. 2008), lung cancer (Rubin et al. 2007), testicular cancer (Argiris et al. 2004), and cervical cancer (Boice et al. 1987; Kleinerman et al. 1995; Chaturvedi et al. 2007). Radiation therapy and alkylating chemotherapy both contribute to the risk, as has been demonstrated in survivors of Hodgkin and non-Hodgkin’s lymphoma (Swerdlow et al. 2001; Travis et al. 2002; Andre et al. 2004). The increased risk seen in survivors of cancers of the head and neck, lungs, and cervix is more likely to be related to tobacco history being a common etiologic factor for both the primary malignancy and the subsequent lung cancer.
The role of low-dose chest CT screening in other high-risk populations has been studied and reported in nonrandomized studies, and the results are conflicting and controversial (Bach 2008; Henschke et al. 2008; Midthun et al. 2008). The National Lung Screening Trial, a randomized study comparing chest X-ray versus chest CT screening in the noncancer population showed a 20 % reduction in lung cancer-specific mortality with chest CT screening (Aberle et al. 2011). The role of chest CT screening in cancer survivors has not been studied. However, for selected survivors who are deemed at high risk based on the their treatment and smoking history, and in whom further radiation therapy is not feasible because of their prior chest irradiation, they may particularly benefit from early detection through a more sensitive screening modality such as CT scan, with the goal of detecting lung cancers at an early, operable, and presumably more curable stage.
Given the known contribution of smoking to lung cancer risk, and the multiplicative effect of smoking on lung cancer risk after cancer therapy (Travis et al. 2002; Kaufman et al. 2008), survivors who continue to be smokers would greatly benefit from smoking cessation counseling and referral to smoking cessation programs.
4.1.3 Skin Cancer as a Second Malignancy
An increased risk of non-melanomatous skin cancer, in particular basal cell carcinoma, within prior radiation treatment fields have been demonstrated (Colman et al. 1988; Levi et al. 2006a). Synchronous or metachronous skin malignancies are also commonly seen in patients who already have a skin cancer diagnosis (Karagas et al. 1996; Goggins et al. 2003; Revenga et al. 2004; Levi et al. 2006b; Titus-Ernstoff et al. 2006; Cardous-Ubbink et al. 2007), as well as in patients with history of non-Hodgkin’s lymphoma (Goggins et al. 2001; Hemminki et al. 2003; Loriot et al. 2006), chronic leukemia (Travis et al. 1992), and in patients status bone marrow or stem cell transplantation (Hasegawa et al. 2005; Cavalier et al. 2006). The increased risk of skin cancer in these patients are likely related to sun exposure and compromised immune status, rather than from prior cancer therapy. In cancer survivors at high risk of developing a skin malignancy, risk-reduction through sun-safety practice, and early-detection through regular self-examination and at least annual skin examination by a dermatologist are essential.
4.1.4 Endometrial Cancer as a Second Malignancy
Tamoxifen is commonly used as adjuvant therapy in women with estrogen-receptor positive breast cancer, and has been shown to reduce the risk of contralateral breast cancer by 30–40 % (Swerdlow et al. 2001). It is also an effective chemoprevention agent, reducing the risk of breast cancer by 50 % in high-risk women (Fisher et al. 1998). However, several large studies have shown that tamoxifen therapy is associated with a 2- to 4-fold significantly increased risk of endometrial caner (Magriples et al. 1993; Fisher et al. 1994; Bergman et al. 2000
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree