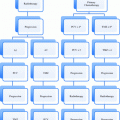
Questions to address
Should a given patient receive radiotherapy, as opposed to for example surgery?
Should we combine radiotherapy with systemic agents?
What is the optimal regimen for a given patient?
What is the true disease extent in the presence of given imaging findings?
What is the biology/aetiology of the tumour?
What do we need to delineate on treatment planning scans?
How does the optimal dose distribution look like?
Is there a role for dose intensification to subvolumes?
Do we need adaptive replanning during radiotherapy?
Can we predict response early during treatment?
Is there a need for salvage surgery?
What is the risk of severe acute toxicity?
Are preventive measures indicated, such as feeding tubes?
What is the risk of significant late toxicity?
What is the anticipated functional outcome and need for rehabilitation?
What is the risk of a second primary tumour?
2 Recent Developments
In the last decades, major progress has been made in the treatment of patients with HNSCC. In particular, the addition of concomitant chemotherapy (Langendijk et al. 2004; Pignon et al. 2000, 2009) to radiation and the introduction of altered fractionation schedules (Bourhis et al. 2006) have resulted in a significant improvement of loco-regional tumour control and overall survival. These new treatment regimens have gained conceptual acceptance and are now considered standard among patients with HNSCC in the organ preservation as well as in the unresectable setting. Moreover, the results of recent studies also indicate that new induction chemotherapy regimens (Hitt et al. 2005; Posner et al. 2007; Vermorken et al. 2007) and the addition of cetuximab to radiation (Bonner et al. 2006) may further improve outcome after non-surgical treatment, at least for selected patients. Since the publication of the results of two prospective randomised studies, an increasing number of patients are now treated with postoperative concomitant chemoradiation instead of postoperative radiotherapy alone (Bernier et al. 2004; Cooper et al. 2004). In this setting extracapsular extension and positive surgical margins are important predictors of loco-regional control and survival.
Human papilloma virus (HPV) status has been shown to predict outcomes (loco-regional control, metastases-free survival, survival) in several series (Fakhry et al. 2008; Lassen et al. 2009; Rischin et al. 2010; Oguejiofor et al. 2013; Rades et al. 2013a). Positivity might be assessed by in situ hybridisation and/or positive p16 immunostaining. In Radiation Therapy Oncology Group (RTOG) studies median pack-years of tobacco smoking were lower among p16-positive than p16-negative patients with oropharyngeal cancer in both trials (RTOG 9003: 29 v 46 pack-years; p = 0.02; RTOG 0129: 10 v 40 pack-years; p < 0.001) (Gillison et al. 2012). After adjustment for p16 and other factors, risk of progression or death increased by 1 % per pack-year (for both, hazard ratio [HR], 1.01; 95 % CI, 1.00 to 1.01; p = 0.002) or 2 % per year of smoking (for both, HR, 1.02; 95 % CI, 1.01 to 1.03; p < 0.001) in both trials. In RTOG 9003, risk of death doubled (HR, 2.19; 95 % CI, 1.46 to 3.28) among those who smoked during radiotherapy after accounting for pack-years and other factors, and risk of second primary tumours increased by 1.5 % per pack-year (HR, 1.015; 95 % CI, 1.005–1.026). Despite the association with more advanced nodal stage, patients with HPV positive oropharyngeal cancers have better outcomes. A study by Hong et al. suggested that the prognostic significance of the conventional staging system in tonsillar cancer is modified by HPV (Hong et al. 2013). Ongoing studies will determine whether or not future treatment strategies should be different for the two patient groups with or without HPV positivity.
3 Prognostic Factors for Survival and Predictors for Recurrence
In general, prognosis depends on the biological aggressiveness of the tumour, e.g. its ability to metastasise and to withstand non-surgical treatment attempts. Host characteristics play an important role because they determine whether aggressive treatment is possible. A large analysis of 1,093 participants in two RTOG trials (9003, 9111) showed that age, performance status, marital status and cigarette smoking significantly predicted survival (Coyne et al. 2007). Prognosis and primary treatment approach vary with anatomical site and disease extent staged according to the AJCC TNM system.
3.1 Nasopharyngeal Cancer
For nasopharyngeal cancer the AJCC TNM staging system is shown in Tables 2 and 3. Besides T category (local invasion), tumour volume is of prognostic significance, especially when attempting to kill cancer cells by radio- and chemotherapy as opposed to complete surgical resection (cell number, increasing likelihood of hypoxia in larger tumours) (Sze et al. 2004; Chen et al. 2009; Guo et al. 2012). Other staging systems have been developed by different groups. In general, T classification predominantly affects local control, while N classification predicts neck and distant control. Epstein-Barr virus DNA (less than versus equal to or greater than 1,500 copies/ml) is a prognostic biomarker that predicts overall and relapse-free survival. Other markers such as serum lactate dehydrogenase (LDH) (Zhou et al. 2012; Wan et al. 2013), vascular endothelial growth factor (VEGF) and osteopontin have been suggested and should be studied further (Lv et al. 2011).
Table 2
AJCC TNM classification of carcinoma of the nasopharynx
Stage | Description |
|---|---|
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor confined to the nasopharynx, or tumor extends to oropharynx and/or nasal cavity without parapharyngeal extension (i.e., posterolateral infiltration of tumor) |
T2 | Tumor with parapharyngeal extension (i.e., posterolateral infiltration of tumor) |
T3 | Tumor involves bony structures of skull base and/or paranasal sinuses |
T4 | Tumor with intracranial extension and/or involvement of cranial nerves, hypopharynx, or with extension to the infratemporal fossa/masticator space |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Unilateral (including ipsilateral) metastasis in cervical lymph node(s), ≤6 cm in greatest dimension, above the supraclavicular fossa, and/or unilateral or bilateral, retropharyngeal lymph nodes, ≤6 cm in greatest dimension |
N2 | Bilateral metastasis in cervical lymph node(s), ≤6 cm in greatest dimension, above the supraclavicular fossa |
N3a | Metastasis in a lymph node(s) >6 cm in dimension |
N3b | Metastasis in a lymph node(s) to the supraclavicular fossa |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis (including seeding of the peritoneum and positive peritoneal cytology) |
Table 3
Stage grouping of carcinoma of the nasopharynx
Stage grouping | ||||
|---|---|---|---|---|
T1 | T2 | T3 | T4 | |
N0 | I | II | III | IVA |
N1 | II | II | III | IVA |
N2 | III | III | III | IVA |
N3 | IVB | IVB | IVB | IVB |
M1 | IVC | IVC | IVC | IVC |
3.2 Cancer of the Oral Cavity and Oropharynx
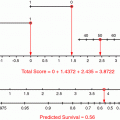
For cancer of the oral cavity and oropharynx the AJCC TNM staging system is shown in Tables 4 and 5. In these diseases, male gender, cigarette smoking and poor performance status are adverse prognostic factors. Patients who are p16 positive have improved survival. After surgical resection for oral cavity carcinoma, adjuvant radiotherapy may be recommended for patients at higher risk for loco-regional recurrence, but it can be difficult to predict whether a particular patient will benefit. Wang et al. constructed several types of survival models using a set of 979 patients with oral cavity squamous cell carcinoma treated in the United States and Brazil (Wang et al. 2013). Covariates were age, sex, tobacco use, stage, grade, margins, and subsite. The best performing model was externally validated on a set of 431 patients from Princess Margaret Hospital, Toronto, Canada. The primary outcome measure of interest was loco-regional recurrence-free survival. An online nomogram was built from this model that estimates loco-regional failure-free survival with and without postoperative radiotherapy. However, none of the patients had received postoperative radiochemotherapy. Information on extracapsular extension, lymphovascular space invasion, and perineural invasion were not available in this data set, also limiting its general use. The prediction model indicated that patients with positive margins and/or N2–N3 disease will derive the most benefit from adjuvant radiotherapy, although the relative magnitude of this benefit will vary depending on the other patient and tumour characteristics entered into the model. Histologic grade was also an independent predictor of prognosis in stage I or II oral cavity carcinoma in the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute (Thomas et al. 2013). Among patients age 20–65 with AJCC stage I or II cancer, the adjusted risk of death was 2.7 times greater (95 % CI 1.7 to 4.1) if the tumour was poorly differentiated or undifferentiated than it was if the tumour was well differentiated. Among patients age 66–94, the risk of death was 3.0 (95 % CI 2.0–4.5) times greater. For those over age 65, moderately differentiated tumours also conferred an estimated 42 % increased risk of death, which was borderline significant (p = 0.05).
Table 4
AJCC TNM classification of carcinoma of the oral cavity and oropharynx
Stage | Description |
|---|---|
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor ≤2 cm in greatest dimension |
T2 | Tumor >2 cm but ≤4 cm in greatest dimension |
T3 | Tumor >4 cm in greatest dimension |
T4 (lip) | Tumor invades through cortical bone, inferior alveolar nerve, floor of mouth, or skin of face, chin, or nose |
T4a (oral cavity) | Tumor invades through cortical bone, into deep (extrinsic) muscle of tongue, maxillary sinus, or skin of face |
T4b (oral cavity) | Tumor involves masticator space, pterygoid plates, or skull base, and/or encases internal carotid artery |
T4a (oropharynx) | Tumor invades the larynx, deep, extrinsic muscle of tongue/medial pterygoid/hard palate, or mandible |
T4b (oropharynx) | Tumor involves lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, skull base, and/or encases internal carotid artery |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
N2a | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
N2b | Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension |
N2c | Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
N3 | Metastasis in a lymph node, >6 cm in greatest dimension |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis (including seeding of the peritoneum and positive peritoneal cytology) |
Table 5
Stage grouping of carcinoma of the oral cavity and oropharynx
Stage grouping | |||||
|---|---|---|---|---|---|
T1 | T2 | T3 | T4a | T4b | |
N0 | I | II | III | IVA | IVB |
N1 | III | III | III | IVA | IVB |
N2 | IVA | IVA | IVA | IVA | IVB |
N3 | IVB | IVB | IVB | IVB | IVB |
M1 | IVC | IVC | IVC | IVC | IVC |
It is hypothesised that molecular biomarkers might improve current decision models. Protein 53 (p53), insulin-like growth factor II mRNA-binding protein 3 (IMP3), cyclooxygenase 2 (COX2), and HuR were analysed by immunohistochemistry in 96 patients with primary oral squamous cell cancer who underwent surgical resection at the Yonsei Dental Hospital in Seoul, Korea (Kim et al. 2012). On univariate and multivariate analysis, the expression of IMP3 was significantly associated with the risk of death. P53 was also significantly associated with survival in the case of negative IMP3 and the prediction accuracy was improved by including these 2 factors in the prediction model. The latter nomogram included age, sex, presence of lymph node metastases, T stage, tumour site, p53 and IMP3. In general, such tools need external validation in sufficiently large databases before widespread implementation.
3.3 Cancer of the Larynx and Hypopharynx
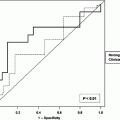
For cancer of the larynx and hypopharynx the AJCC TNM staging system is shown in Table 6. Egelmeer et al. performed a population-based cohort study on 994 laryngeal carcinoma patients, treated with radiotherapy from 1977 until 2008 (Egelmeer et al. 2011). Two nomograms were developed and validated. Performance of the models was expressed as the area under the curve (AUC). Unfavourable prognostic factors for overall survival were low haemoglobin level, male sex, high T status, nodal involvement, older age, lower EQD(2T) (total radiation dose corrected for fraction dose and overall treatment time), and non-glottic tumour. All factors except tumour location were predictive for local control. The AUCs were 0.73 for overall survival and 0.67 for local control. External validation of the survival model yielded AUCs of 0.68, 0.74, 0.76 and 0.71 for cohorts from Leuven (n = 109), VU Amsterdam (n = 178), Manchester (n = 403) and Amsterdam (n = 205), respectively, while the validation procedure for the local control model resulted in AUCs of 0.70, 0.71, 0.72 and 0.62. The resulting nomograms were made available on the website www.predictcancer.org. Further evidence from multivariate analyses suggests that tumour volume also influences local control and survival in T2 tumours (Rutkowski et al. 2013) and T3-4 tumours (Hoebers et al. 2013).
Table 6
AJCC TNM classification of carcinoma of the larynx and hypopharynx
Stage | Description |
|---|---|
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
Supraglottis | |
T1 | Tumor limited to 1 subsite of supraglottis, with normal vocal cord mobility |
T2 | Tumor invades mucosa of more than 1 adjacent subsite of supraglottis or glottis or region outside the supraglottis, without fixation of the larynx |
T3 | Tumor limited to larynx with vocal cord fixation and/or invades any of the following: postcricoid area, preepiglottic space, paraglottic space, and/or inner cortex of thyroid cartilage |
T4a | Moderately advanced local disease: Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx |
T4b | Very advanced local disease: Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
Glottis | |
T1a | Tumor limited to 1 vocal cord (may involve anterior or posterior commissure) with normal mobility |
T1b | Tumor involves both vocal cords (may involve anterior or posterior commissure) with normal mobility |
T2 | Tumor extends to supraglottis and/or subglottis, and/or with impaired vocal cord mobility |
T3 | Tumor limited to the larynx with vocal cord fixation and/or invasion of paraglottic space, an/or inner cortex of the thyroid cartilage |
T4a | Moderately advanced local disease: Tumor penetrates the outer cortex of the thyroid cartilage and/or invades tissues beyond the larynx |
T4b | Very advanced local disease: Tumor invades prevertebral space, encases carotid artery, or involves mediastinal structures |
Subglottis | |
T1 | Tumor limited to the subglottis |
T2 | Tumor extends to vocal cord(s) with normal or impaired mobility |
T3 | Tumor limited to larynx with vocal cord fixation |
T4a | Moderately advanced local disease: Tumor invades cricoid or thyroid cartilage and/or invades tissues beyond the larynx |
T4b | Very advanced local disease: Tumor invades prevertebral space, encases carotid artery, or involves mediastinal structures |
Hypopharynx | |
T1 | Tumor limited to 1 subsite of hypopharynx and/or ≤2 cm in greatest dimension |
T2 | Tumor invades more than 1 subsite of hypopharynx or an adjacent site, or measures >2 cm but ≤4 cm in greatest dimension |
T3 | Tumor >4 cm in greatest dimension or with fixation of hemilarynx or extension to esophagus |
T4a | Moderately advanced local disease: Tumor invades thyroid/cricoid cartilage, hyoid bone, thyroid gland, or central compartment soft tissue includes prelaryngeal strap muscles and subcutaneous fat |
T4b | Very advanced local disease: Tumor invades prevertebral fascia, encasescarotid artery, or involves mediastinal structures |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
N2a | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
N2b | Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension |
N2c | Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
N3 | Metastasis in a lymph node, >6 cm in greatest dimension |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Wang et al. (2013) analyzed the impact of the lymph node ratio (LNR, ratio of metastatic to examined nodes) on the prognosis of hypopharyngeal cancer patients. SEER registered hypopharyngeal cancer patients with lymph node metastasis were evaluated using multivariate Cox regression analysis to identify the prognostic role of the LNR. The categorical LNR was compared with the continuous LNR and pN classifications to predict cause-specific and overall survival (n = 916). T classification, N classification, M classification, the number of regional lymph nodes examined, the continuous LNR (Hazard ratio 2.4, 95 % CI 1.7–3.4, p < 0.001) were among prognostic variables that were associated with cause-specific survival. The categorical LNR showed a higher C-index and lower Akaike information criterion (AIC) value than the continuous LNR. When patients were classified into four risk groups according to LNR, R0 (LNR = 0), R1 (LNR ≤ 0.05), R2 (LNR 0.05–0.3) and R3 (LNR > 0.3), the Cox regression model for both endpoints using the R classification had a higher C-index value and lower AIC value than the model using the pN classification. In conclusion, using the cutoff points 0.05/0.3, the R classification was more accurate than the pN classification in predicting survival.
4 Haemoglobin and Oxygenation
Tumour perfusion and oxygenation have long been of interest in head and neck cancer research. Identification of potential surrogate markers and dynamics during treatment are subject of numerous recent studies. Low haemoglobin is associated with inferior loco-regional control and survival in multivariate analyses of mixed populations (Rades et al. 2013b: stage III/IV, postoperative radiotherapy; McCloskey et al. 2009: definitive chemoradiation; Agarwala et al. 2007: definitive chemoradiation). Correction of pre-treatment low haemoglobin by blood transfusion and/or erythropoietin (EPO) stimulating agents does, however, not improve the outcome (Hoff 2012). Smoking leads to a decrease in effective haemoglobin and poorer treatment outcome. Smoking should be avoided in order to improve the therapeutic efficacy of radiotherapy and development of other smoking-related diseases and/or secondary cancers. A study on postoperative radiotherapy with multivariate analysis suggested that improved loco-regional control was significantly associated with no EPO expression of tumour cells (risk ratio [RR] 3.7; 95 % CI 1.35–15.4; p = 0.008) (Seibold et al. 2013). Improved metastases-free survival was also significantly associated with no EPO expression (RR 5.45; 95 % CI 1.1–97.8; p = 0.031). The same holds true for improved survival.
The concentration of osteopontin (SPP1) in plasma is associated with tumour hypoxia. The Danish Head and Neck Cancer group DAHANCA 5 trial found that the hypoxia radiosensitiser nimorazole significantly improved the outcome of radiotherapy for patients with head and neck cancer compared with placebo. However, whether all patients benefit from such modification of hypoxia is unclear. DAHANCA researchers aimed to assess whether the concentration of plasma osteopontin could predict response to the hypoxia radiosensitiser in 320 patients randomised in the DAHANCA 5 trial (Overgaard et al. 2005). Samples were grouped into tertiles according to high, intermediate or low concentrations of plasma osteopontin, and analysed for loco-regional tumour control and disease-specific survival at 5 years. Overall, loco-regional tumour failure and disease-specific mortality were more frequent in patients assigned placebo than in those assigned nimorazole. Loco-regional tumour failure was more frequent in patients with high concentrations of osteopontin assigned placebo than in those with high concentrations assigned nimorazole, as was disease-specific mortality. However, neither loco-regional tumour failure nor disease-specific mortality differed between groups for patients with low concentrations of plasma osteopontin or for those with intermediate concentrations. This study suggested that high plasma concentrations of osteopontin are associated with a poor outlook after radiotherapy for patients with head and neck cancer, but can be improved by use of nimorazole.
TROG researchers sought to confirm the prognostic and predictive significance of osteopontin in patients treated on a large international trial (stage III/IV, randomised to receive definitive radiotherapy concurrently with cisplatin or cisplatin plus the hypoxic cell cytotoxin tirapazamine (n = 578) (Lim et al. 2012). Osteopontin concentrations were analyzed for overall survival and loco-regional failure, adjusting for known prognostic factors. Additional analysis was carried out in patients with available tumour p16 staining status. High osteopontin levels were not associated with worse outcome. There was no interaction between osteopontin and treatment arm for either endpoint. Thus, there is no conclusive evidence that high plasma osteopontin levels are associated with an adverse prognosis, or are predictive of benefit with hypoxia targeting therapy. The same TROG dataset suggested that elevated plasma interleukin (IL)-8 level is an independent prognostic factor for survival irrespective of treatment (Le et al. 2012).
A different study from the Netherlands (based on a randomised trial in patients with laryngeal cancer) reported that only in tumours with a low EGFR fraction (immunohistochemical staining), adding hypoxia modification to accelerated radiotherapy has an additive beneficial effect on outcome (Nijkamp et al. 2013). EGFR expression appears to be a predictive biomarker for the selection of patients that will or will not respond to carbogen and nicotinamide (ARCON), a hypoxia targeting strategy that is thought to counteract enhanced tumour cell proliferation- and hypoxia-related radioresistance. Hypoxia gene expression signatures are another developing strategy to assess and categorise hypoxia (Toustrup et al. 2012). This method has evolved along with the development of complementary DNA microarray analysis and classifies tumours in accordance to the expression of specific hypoxia-responsive genes in the tumour biopsy. Thus, tumours are classified and categorised in terms of the biological behaviour to hypoxic conditions in the microenvironment. Ongoing research will determine the clinical applicability of gene expression signatures, also with regard to other endpoints such as normal tissue toxicity.
A recent DAHANCA study evaluated (18)F-fluoroazomycin arabinoside (FAZA) positron emission tomography (PET)/CT hypoxia imaging as a prognostic factor in HNSCC patients receiving radiotherapy (Mortensen et al. 2012). Forty patients were included. Static FAZA PET/CT imaging was conducted prior to irradiation. The hypoxic volume (HV) was delineated. In 13 patients, a repetitive FAZA PET/CT scan was conducted during the radiotherapy treatment. A hypoxic volume could be identified in 25 (63 %) of the 40 tumours. FAZA PET HV varied considerably with a range from 0 to 31 (median: 0.3) cm3. The distribution of hypoxia among the HPV positive and negative tumours was not significantly different. In the FAZA PET/CT scans performed during radiotherapy, hypoxia could be detected in six of the 13 patients. For these six patients the location of HV remained stable in location during radiotherapy treatment, though the size of the HV decreased. In 30 patients a positive correlation was detected between maximum FAZA uptake in the primary tumour and the lymph node. During a median follow up of 19 months a significant difference in disease-free survival rate with 93 % for patients with non-hypoxic tumours and 60 % for patients with hypoxic tumours could be detected. The definitive role of FAZA PET/CT imaging as a suitable assay with prognostic potential for detection of hypoxia should be determined in confirmatory trials. Other tracers and imaging methods (dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), DCE computed tomography, and diffusion-weighted MRI, measuring for example distribution of tumour blood volume and blood flow) are also under prospective evaluation (Quon and Brizel 2012).
5 Other Aspects of PET/CT
PET/CT might improve staging of HNSCC and is recommended in the M and bilateral nodal staging of all patients where conventional imaging is equivocal, or where treatment may be significantly modified (Yoo et al. 2013). PET is recommended in all patients after conventional imaging and in addition to, or prior to, diagnostic panendoscopy where the primary site is unknown (Nieder et al. 2001). Besides staging and target volume delineation, other advantages of PET have been discussed in the literature [prognostic and predictive information, monitoring of response, adaptive radiotherapy (Garg et al. 2012)]. All these potential indications require additional evidence from prospective trials. However, examples from the literature are presented here. Metabolic tumour volume (MTV) of (18)F-FDG PET/CT is a volumetric measurement of tumour cells with increased 18F-FDG uptake. Park et al. evaluated the prognostic value of MTV in 81 patients with locoregionally advanced laryngeal and hypopharyngeal cancer (Park et al. 2013). On multivariate analysis, MTV was an independent prognostic factor for both loco-regional control and survival. Comparable findings were made by Tang et al. in 83 patients (Tang et al. 2012). A different study included 69 patients with SCC of the tonsil who underwent pre-treatment FDG PET/CT with measurement of maximum standardized uptake value (SUV(max)), MTV, total lesion glycolysis (TLG), and asymmetry indices (of SUV(max), MTV, and TLG) (Moon et al. 2013). The prognostic significance of these parameters and clinical variables was assessed by multivariate Cox proportional hazards regression analysis with adjustments for age, sex, and AJCC stage. This study showed that only TLG (HR 1.02, 95 % CI 1.003–1.037, p = 0.023) was an independent prognostic factor associated with decreased overall survival.
An ongoing multi-centre trial aims to improve outcome in two ways. Firstly, by redistribution of the radiation dose to the metabolically most FDG avid part of the tumour (Heukelom et al. 2013). Hereby, a biologically more effective dose distribution might be achieved while simultaneously sparing normal tissues. Secondly, by improving patient selection. Both cisplatin and EGFR antibodies like cetuximab in combination with radiotherapy are effective in enhancing tumour response. However, it is unknown which patients will benefit from either agent in combination with irradiation. The plan is to analyse the predictive value of biological markers and (89)Zr-cetuximab uptake for treatment outcome of chemoradiation with cetuximab or cisplatin to improve patient selection. The so-called ARTFORCE study is a randomized phase II trial for 268 patients with a factorial 2 by 2 design: cisplatin versus cetuximab and standard versus redistributed radiotherapy. Adaptation of treatment for anatomical changes in the third week of treatment is planned. Patients with locally advanced squamous cell carcinoma of the oropharynx, oral cavity or hypopharynx are eligible. Primary endpoints are: loco-regional recurrence-free survival at 2 years, correlation of the median (89)Zr-cetuximab uptake and biological markers with treatment specific outcome, and toxicity (clinicaltrials.gov, identifier: NCT01504815).