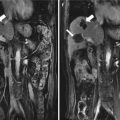
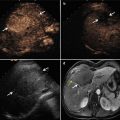
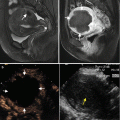
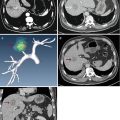
Fig. 13.1
Images in a 58-year-old man with 1.8 cm hepatocellular carcinoma treated by percutaneous microwave ablation (MWA) with artificial pleural effusion. (a) Contrast-enhanced magnetic resonance imaging (MRI) before MWA shows the neoplasm appearing in hyper-enhancement (small arrow) on arterial phrase and hypo-enhancement (large arrow) on venous phrase. (b) Left sonogram shows the puncture of the pleural cavity with a 16-gauge BD angiocath needle (small arrow). The middle sonogram shows the hyper-enhanced nodule on contrast-enhanced ultrasound, which is unclear on grey scale (large arrow). (c) Sonogram shows the procedure of MWA. The ablating area appears as a hyper-echoic region (large arrows) with artificial pleural effusion surrounded on grey-scale (small arrows). (d) Contrast-enhanced MRI 1 month after MWA shows the ablation zone appearing unenhanced in the arterial phrase (small arrow) and the venous phrase (large arrow)


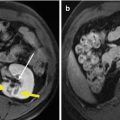
Fig. 13.2
Images in a 58-year-old man with 2.6 cm hepatocellular carcinoma treated by percutaneous MWA with artificial pleural effusion. (a) Contrast-enhanced MRI before MWA shows the neoplasm appearing as a high signal on T2WI (small arrow) and in hyper-enhancement (large arrow) on arterial phrase. (b) Left sonogram shows the puncture of the pleural cavity with a 16-gauge BD angiocath needle (small arrow). The middle sonogram shows the hyper-enhanced nodule on contrast-enhanced ultrasound (large arrow) with the artificial pleural effusion surrounded (triangle). (c) Sonogram shows the procedure of MWA. The ablating area appears as a disturbed blood flow on color-Doppler (small arrow) for microwave emitting, and as a hyper-echoic region on grey-scale (large arrow). (d) Contrast-enhanced MRI 1 month after MWA shows the ablation zone appearing as a low signal on T2WI (small arrow) and unenhanced in the arterial phrase (large arrow)
13.7 Efficacy of Percutaneous MWA with Artificial Pleural Effusion
To examine the efficacy of percutaneous MWA with artificial pleural effusion, we designed a case-control study, and compared the primary technique effectiveness rate, local tumor progression rate, and tumor-free survival rate between an artificial pleural effusion group and a control group which was matched in terms of tumor differentiation, tumor size, and tumor location. In previous studies [1, 3], tumor differentiation and tumor size were chosen as the independent prognostic factors affecting recurrence of hepatocellular carcinoma after microwave ablation treatment. Therefore, tumor differentiation and tumor size were selected as a matching standard to make sure that the two groups were balanced as far as possible. In addition, we matched tumor location between the two groups to compare complications after treatment. The results of our study showed that the primary technique effectiveness rate, the 1-, 2-, and 3-year local tumor progression rates, and the 1-, 2-, and 3-year tumor-free survival rates in the two groups had no significant difference. Therefore, percutaneous MWA with artificial pleural effusion for liver tumors located in the hepatic dome has a similar therapeutic effect to that of percutaneous MWA for liver tumors with good ultrasonic visibility.
13.8 Conclusion
Artificial pleural effusion could be a feasible, safe, and effective assistive technique for the expansion of indications of microwave or radiofrequency ablation for liver tumors located in the hepatic dome.
References
1.
2.
N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrie N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M, Seror O. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50(5):1475–83.PubMedCrossRef
3.
4.
Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–7.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree