, Xiao-ling Yu1 , Ping Liang1, Zhigang Cheng1, Zhiyu Han1, Fangyi Liu1 and Jie Yu1
(1)
Department of Intentional Ultrasound, Chinese Liberation Army General Hospital, 28 Fuxing Road, Beijing, 100853, China
Abstract
Image-guided percutaneous ablation therapy for hepatocellular carcinoma has been used worldwide, and the most widely used modalities are radiofrequency ablation (RF) and microwave ablation (MA). Multiphasic contrast-enhanced CT, dynamic MRI, and contrast-enhanced ultrasound are accepted as reliable modalities for evaluating the adequacy of radiofrequency and microwave ablation and early detection of tumor recurrences. In this session, we evaluate the clinical utility of low mechanical index contrast-enhanced ultrasound in assessing percutaneous microwave ablation response in patients with hepatocellular carcinoma by comparing the results to contrast-enhanced magnetic resonance imaging and prove that contrast-enhanced ultrasound examination is a safe and easy modality in assessing therapeutic effect of microwave ablation.
Keywords
Hepatocellular carcinomaMicrowave ablationContrast-enhanced ultrasoundMagnetic Resonance Imaging30.1 Introduction
Hepatocellular carcinoma is the second leading cause of death with more than 200,000 victims each year in China. Image-guided percutaneous ablation therapy for hepatocellular carcinoma has been used worldwide, and the most widely used modalities are radiofrequency ablation and microwave ablation [1–4]. The success of ablative treatment is strongly dependent on complete tumor destruction, which requires both accurate placement of ablative probes in the lesion and thorough understanding of the ablative range [5, 6]. In order to evaluate the therapeutic effect and to detect residual tumor, much effort has been made on the identification of an imaging technique that can permit early localization of surviving tumor tissue post therapy.
30.2 Application Status
Nowadays ultrasound has been widely used as an effective imaging modality to guide the treatment due to its real-time, easiness of use, noninvasiveness, and lack of ionizing radiation. However, conventional ultrasound is of little help in the assessment of both pretreatment tumor vascularity and post-treatment therapeutic response. Assessment of tissue perfusion is crucial to differentiate necrotic from viable residual tumor. CEUS has been used in some locoregional therapies, which conventionally include ablation and transarterial chemo-/radioembolization, and plays a key role in the management of patients with liver malignancies, both HCC and metastases. At present, most studies have used CEUS or CEMRI/CECT to assess the efficacy of ablation treatment. The enhancement patterns observed during the arterial, portal venous, and late phases are generally similar among CEUS, CECT, and CEMRI. The real-time nature of CEUS allows depiction of early arterial phase enhancement which is sometimes missed on CT and MRI because they have lower frame rates. Discordance has also been shown in some lesions during the portal venous and late phases when CT and MRI contrast materials diffuse into the tumor interstitium and may conceal washout. On the other hand, postvascular phase imaging with Sonazoid® (made by Schering AG) shows patterns similar to those described with superparamagnetic iron oxide (SPIO) MRI. The aims of application of CEUS are as follows: (1) assessment of the lesions to be treated by ablation (number, size and enhancement characteristics of the lesions, and the presence of feeding vessels) to define the eligibility of the patient for treatment and the best ablation strategy, (2) depicting undetectable lesions under conventional US and guiding probe puncture, and (3) detecting viable tumor following locoregional treatment (either ablation or chemo-/radioembolization). In this section, we will discuss this in more detail below, focusing primarily on this part.
30.3 Periprocedural Assessment of Treatment Response
In the pretreatment stage, the assessment of tumor size must include the perilesional hypervascular halo. Tumor margins are better detected by CEUS than conventional US because definition of its relationships with surrounding structures is improved; thus, CEUS can help define the size and margin and the nourishing blood vessels of the lesion to develop appropriate treatment strategies and reduce the risks of complications. Hence, pretreatment CEUS is essential for comparison of the patterns before and after treatment.
In the follow-up investigation to assess tumor recurrence stage, it is often difficult to depict local tumor recurrence after ablation using conventional US alone. Here, scanning in the late or postvascular phase of the lesion, with subsequent reinjection of contrast agent to confirm tumor enhancement in any suspicious region, is useful to identify the viable tumor adjacent to the ablated volume. This can be used to guide biopsy and complementary treatment. While CEUS may be extremely useful to define local recurrence in a treated nodule, CT and MRI provide a better overview of the liver to detect distant intra- and extrahepatic tumor and cannot be replaced by CEUS.
Therapeutic effect was assessed by the result of MRI after ablation, the proved serum tumor marker levels, and additional follow-up. Completely treated lesions show no contrast enhancement by dynamic CT or MRI, which indicates the disappearance of blood flow in the lesion. If the treatment is incomplete, the residual tumor is detected by contrast enhancement in the treated area [14]. The criteria used for determining tumor response to ablation treatment in CEMR imaging during follow-up period were (1) complete tumor necrosis if no foci of enhancement were seen within and in the peripheral ablated area on CEMR imaging [14] or (2) presence of residual, incompletely treated tumor, if during the CEMRI arterial phase, the presence of a hyper-dense area was seen, becoming progressively iso- and hypo-dense with respect to the surrounding parenchyma. For CEUS, when a lesion had the appearance of enhancement in the hepatic arterial phase lesion and was identified within or adjacent to the treated area during the follow-up, it was defined as a local recurrence. Two experienced radiologists who were blinded to clinical and imaging information of the patients and not involved in the CEMRI scan or ablative treatment procedure evaluated the findings of post-treatment CEMRI in consensus. CEUS digital clips were retrospectively analyzed in consensus by two experienced sonographers who were blinded to the other imaging information of the patients.
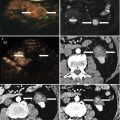
In the early postablative evaluation (within the first 30 days), a thin, uniform enhancing rim can be visible along the periphery of the necrotic region, similar to the findings on CECT or CEMR. It could be considered as the perilesional congestion zone and the misinterpretation of it as residual viable tumor needs to be avoided. The imaging indicator of complete ablation is the disappearance of any previously visualized intralesional enhancement on CEUS. This must be assessed throughout the whole volume of each tumor which has undergone ablation. The volume of the necrosis achieved should be compared with the pretreatment volume of the tumor(s). Simultaneous display of tissue and contrast is of particular value for follow-up of treated lesions. The volume of necrosis achieved can be compared with the pretreatment volume of the same lesion on CECT or CEMRI using real-time fusion imaging (Figs. 30.1 and 30.2).



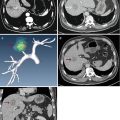
Fig. 30.1
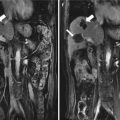
The patient is male, 73 years old, diagnosed as with hepatocellular carcinoma (HCC) (3.3 × 2.2 cm) for 1 month, underwent the contrast-enhanced ultrasound (CEUS) and contrast-enhanced magnetic resonance (CEMR) before the operation. After the necessary examination, the patient underwent microwave ablation (MA). Both the CEUS and the CEMR showed recurrence of the lesion after 2 months, and the patient was treated by microwave ablation again. (a) Hypoechoic lesion in the right lobe of the liver on B-mode sonography and the blood signals of the lesion on CDFI. (b) Intratumoral enhancement in the early arterial phase of CEUS. (c) The presence of residual carcinoma showed enhancement in the arterial phase of CEUS (red arrow showed ablative zone; green arrow showed residual carcinoma). (d) MRI T2WI shows a hyperintense nodule (green arrow) beside the ablative area (green arrow)

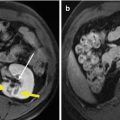
Fig. 30.2
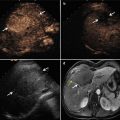
The patient is male, 57 years old, diagnosed as with HCC (3.5 × 2.5 cm) for 2 months. He underwent MA, and after 1 month, the CEUS showed no recurrence, but the CEMR showed recurrence. The pathology result showed there was no recurrence. (a) Intratumoral enhancement in the early arterial phase of CEUS before ablation. (b) Hypoechoic lesion compared to the peripheral liver tissue in the late phase of CEUS before ablation. (c) It showed no enhancement in the lesion in arterial phase of CEUS after being treated for 1 month. (d) MRI T2WI image shows a hyperintense nodule beside the ablative area (green arrow)
30.4 Recommended Uses and Indications
CEUS can be used as a new method as CECT and/or CEMRI for pretreatment staging and assessment of target lesion vascularity. Also, it could be used in the evaluation of the immediate treatment effect after ablation and guidance for immediate re-treatment of residual unablated tumor and in the assessment of local tumor progression when follow-up CECT or CEMRI is contraindicated or not conclusive. In addition to CECT and/or CEMRI, CEUS may be used in follow-up protocols. All patients should undergo conventional ultrasound, CEUS, contrast-enhanced CT, and/or gadolinium-enhanced magnetic resonance imaging (MRI) to delineate the target tumor before microwave ablation. Baseline liver assessment should be performed in each patient using conventional gray-scale ultrasound and color/power Doppler to evaluate and record the number, location, size, shape, border, and internal echogenicity of the lesion and the intralesional blood supply. The measurements of maximum diameters for the index tumors are performed on CEUS image during the portal venous phase before the MW ablation because the necrotic zone in this phase is avascular. To determine if the persistence of tumor was present, ablated lesions were assessed in all vascular phases. CEUS examinations were carried out using low mechanical index (MI ≤ 0.2) contrast dedicated methods – contrast pulse sequencing [CPS – Siemens, Mountain View, CA, USA]. The contrast agent used in this study was SonoVue (Bracco, Milan, Italy). It consisted of an aqueous suspension of phospholipid-stabilized sulfur hexafluoride (SF6) gas microbubbles supplied as a lyophilized powder [7, 8]. Contrast-enhanced images (all vascular phases) were acquired digitally on the hard disk of the US system in addition to the continuous imaging on digital video tape and were evaluated in consensus by two expert doctors who had at least 5 years experience using CEUS and were blinded to clinical and imaging information of the patients [9–12]. All MR studies were carried out with the same 1.5 T unit (signal Echo-speed, GE Medical Systems), contrast medium (Magnevist, Schering; 0.1 mmol/kg body weight), and sequences. According to at least two modalities of contrast imaging, all lesions were hypervascular. Histological diagnosis of all lesions was obtained by ultrasound-guided tumor biopsy using an 18-gauge needle in all patients. In patients with multiple nodules that had the same manifestation on contrast-enhanced images, at least one biopsy was performed and they were definitely diagnosed by follow-up.
30.5 Results
Until now, there are few studies that have compared the imaging features and the roles of contrast-enhanced ultrasound and contrast-enhanced MRI in assessing therapeutic response of HCC after microwave ablation. According to our latest study data, a total of 182 patients with 231 lesions diagnosed as suspected or known hepatic malignancies were enrolled Table 30.1. The final diagnosis was confirmed by MRI, serum tumor marker levels, and additional follow-up. In 1 month after ablative treatment, 179/231 (77.5 %) lesions had obtained complete necrosis and 52/231 (22.5 %) lesions had not been treated completely (Tables 30.2 and 30.3); 213/231 (92.2 %) lesions had same diagnostic results by these two imaging methods. 175/231 (75.8 %) had showed complete tumor necrosis both in CEUS and CEMRI. The concordance between CEUS and CEMRI on the presence of residual carcinoma vascularization was observed in 38 patients. While in six patients residual carcinoma vascularization was detected only by CEMR and not by CEUS, in other seven patients, residual carcinoma vascularization was detected by CEUS and not by CEMRI. One patient with residual carcinoma vascularization was not detected by either CEUS or CEMRI, but residual vascularization was shown by CEUS 3 months later and by CEMRI 6 months later. In our study, the sensitivity in evaluating the therapeutic effect of malignant hepatic masses with CEUS and CEMRI was 86.5 % vs. 84.6 %, the specificity 98.3 % vs. 98.9 %, and the accuracy 95.7 % vs. 95.7 %. There was no significant statistical disparity between CEUS and CEMRI (P = 0.01). The sensitivity has been improved greatly to 98.1 % and the specificity has a slight decrease to 97.2 % by a combination of CEUS and CEMRI in the evaluation of the therapeutic effect of malignant hepatic masses (P > 0.05) (Tables 30.4 and 30.5). In the ROC curve, combination of these two methods has a larger AUC (area under the curve) (Area = 0.991) than that of CEUS (Area = 0.953) (P < 0.05) and CEMRI (Area = 0.936) (P < 0.05) (Fig. 30.3). It means that the combination of CEUS and CEMRI is superior to CEUS or CEMRI alone in the evaluation of the therapeutic effect after microwave ablation.
Table 30.1




Baseline clinical characteristics of our patients
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree