Agent
Wavelength (in nm)
Porfimer sodium (Photofrin)
630
2-[1-hexyloxyethyl]-2-devinylpyropheophorbide-a (HPPH)
665
Temoporfin (m-tetrahydroxyphenylchlorin, Foscan)
652
Talaporfin (mono-(L)-aspartylchlorin-e6, NPe6)
664
Endobronchial Lung Cancer
Photodynamic therapy (PDT) treatment of non-small lung cancer (NSCLC) is primarily employed as an endobronchial therapy to treat patients in basically two settings: (a) advanced disease with central airway obstruction, to palliate symptoms and (b) occult central airway tumors that were detected and evaluated using photodynamic diagnosis (PDD) and other endoscopic diagnostic modalities [5]. Current indications of endobronchial PDT are summarized in Table 29.2. Common contraindications and disadvantages are those of other endobronchial procedures. The specific ones are listed in Table 29.3.
Table 29.2
Current PDT options in NSCLC
Localized/early stage (non-metastatic) | Advanced (metastatic/non-surgical) |
|---|---|
Occult central airway carcinoma | Palliation of central airway obstruction |
Recurrent (metachronous) central tumors after definitive therapy. | Neoadjuvant therapy to improve performance status to convert non-surgical candidates into operable |
Reduce extent of surgical resection | Palliation of hemoptysis |
Table 29.3
Contraindications and disadvantages
Contraindications | Disadvantages |
|---|---|
Tumors adjacent to major blood vessel | Skin photosensitivity |
Tracheo-bronchoesophageal fistula | Cannot be made as emergency procedure |
PDT for Palliative Treatment
Endoscopic palliative treatment in advanced lung cancer patients has a very important role. Because of the poor prognosis and often poor performance status, management of these patients is quite complicated and improving their quality of life may require a multidisciplinary approach [6]. PDT has been used in combination with chemo or radiotherapy without increasing the toxicity of these therapies to treat airway obstruction and recurrent hemoptysis [7, 8].
The typical PDT procedure can be done on an outpatient basis but we prefer to perform it as an inpatient setting because of the usual need to “clean-up” procedures. As shown in Video 29.1, we follow the procedure guidelines as found elsewhere [9]. The steps are outlined in Fig. 29.1.
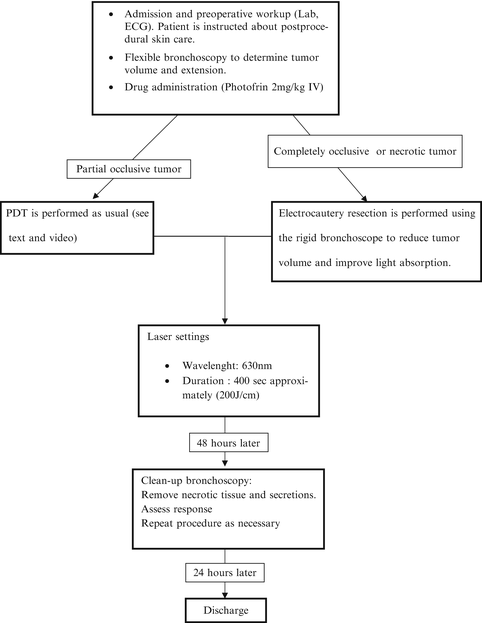

Fig. 29.1
Steps of typical PDT procedure for main stem bronchus obstruction
We mostly use rigid bronchoscopy under general anesthesia as part of the occluding tumor can be removed using electrocautery and core resection, particularly when necrotic tissue is visible. We did not see any complications due to the immediate PDT after electrofulguration of the gross part of the tumor; even better, this resection may enhance light penetration. The laser fiber is introduced via a steerable bronchoscopic laser guide adjacent or even inside the tumor. After the fiber was properly placed, laser activation of the drug is performed. Operating room dim lights are dimmed to avoid skin lesions. During the procedure, laser fiber movement should be kept to a minimum and be checked frequently by turning off the laser. Usual laser safety measures are used like proper glasses. After the laser dose is completed, debris and secretions are aspired. The patient is then sent to the surgery ward where vital signs are monitored. Supplemental humidified oxygen and mucolytic drugs are provided as usually. In case of difficult clearance of secretions, respiratory physical therapy is started.
Two days after initial treatment a new rigid bronchoscopy is performed and PDT is repeated as needed. Again, we prefer the easier management of necrotic tissue and mucous plugs provided by larger-bore rigid instruments. The day after this second bronchoscopy, the patient is discharged if another clean-up procedure is not needed. Follow-up procedures are scheduled in an individual basis and outcome is assessed by patient interview about symptoms, flow-volume spirometry, and flexible bronchoscopy under local anesthesia. Prompt referral to clinical oncology for base treatment is mandatory.
Special Issues
Life-threatening situations like critical airway obstruction and postobstructive pneumonia cannot be treated by PDT because of its delayed mechanism of action. In these cases we prefer to perform electrocautery resection, start antibiotics, and schedule PDT when the patient has overcome the acute episode. Many reports have been published comparing effectiveness of PDT and Nd-YAG laser therapy for endoluminal airway obstruction [10–12] and their results are contradictory, but I think that PDT and thermal ablation (Nd-YAG, electrocautery) are complemental, as shown in the video. Moreover, in our experience and other reports, electrocautery is more cost-effective and can be used during the same procedure [13], unlike Nd-YAG [9].
PDT for Early-Stage Central Lung Cancer
Since the decade of 1980, a growing interest in treating early-stage carcinomas of the central airways has been developed since the initial use of photodynamic diagnosis (PDD). Early clinical experience with PDD was published by Kato, Cortese and Lam working with hematoporphyrin-induced fluorescence [14, 15]. Since then, several autofluorescence devices (AFB) for observing bronchial mucosa allowed the detection of precancerous lesions as severe dysplasia and preinvasive lesions (carcinoma in situ [CIS]) in patients with sputum cytological atypia. Different techniques of illumination have been developed and all proved better than white light alone in detecting early-stage carcinomas [16–18]. The use of different device across published studies produced equivalent results [19].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree