Why Imaging?
Pediatric patients with soft tissue musculoskeletal masses encompass a wide array of pathology that ranges from benign fatty masses and self-involuting vascular tumors all the way to aggressive malignant lesions that require a multidisciplinary approach and staging before intervention is performed. Anesthesia and surgical procedures in children for histological diagnosis before imaging is performed is not without risk both in the short term from procedural complications and also potentially in the long term with the concern of long-term effect of neurological development in children secondary to certain anesthetics. Appropriate imaging not only gives the anatomic extent of the lesion and relationship to vital adjacent structures but also many times provides valuable insights into the composition and aggressiveness of the lesion, which may provide a diagnosis or greatly narrow the differential diagnosis before a procedure is decided.
Who Needs Imaging?
Patients who have skin manifestations of a soft tissue mass that are characteristic and diagnostic do not need further imaging unless the lesion is associated with deep invasion or ill-defined margins, such as kaposiform hemangioendothelioma (KHE), or if the lesions are felt to be metastatic from a primary source, such as a cutaneous manifestation of neuroblastoma. Typically, patients who do not have a classic history for an identifiable benign cutaneous manifestation will benefit from imaging. In many cases, imaging will be diagnostic or at the very least will result in a differential diagnosis. In addition, imaging allows for anatomic detail regardless of which modality, which can be extremely helpful in procedural and surgical planning and also allow for a much higher degree of procedural safety.
Problem Solving: Which Modality? Which Protocol?
Radiographs have a limited role in soft tissue tumor evaluation in children, but in isolated circumstances can give valuable information. In addition, several advantages, such as ease of ordering and availability, the low cost, the minimal amount of ionizing radiation, and the lack of sedation need, are reasons that radiographs may be obtained in the setting of a soft tissue mass.
Ultrasound (US) is readily available and is typically the first imaging modality used to assess a soft tissue mass. US allows for a noninvasive assessment of the anatomy and for the evaluation of flow dynamics, all without exposure to ionizing radiation or the use of intravenous contrast agents. In addition, grayscale and color and spectral Doppler US may be performed as a bedside or outpatient procedure, precluding the need to transport developmentally disabled, critically ill, and unstable patients into unfamiliar beds. In addition, this method provides high spatial resolution imaging of the small vessels, calcifications, and cysts at a relatively low cost. US, however, is operator dependent and may be particularly challenging to perform and interpret in younger children. Overall, vascularity is also difficult to compare and quantify given the subjective nature and the differences in technology between different settings and machines.
Magnetic resonance imaging (MRI), with its high soft tissue contrast, is the current imaging gold standard for the evaluation of soft tissue masses in children, defining lesion diagnostic characteristics while delineating its anatomic relationships with adjacent structures. Magnetic imaging angiography (MRA) permits the noninvasive evaluation of the pediatric soft tissue masses. Some of the advantages of traditional angiography can be obtained today with MRA by using time-resolved contrast-tracking techniques. MRA still does not approach the spatial resolution and accuracy of CTA or traditional angiography, which is rarely used for diagnostic purposes today in pediatrics and is used only in conjunction with a combined planned therapeutic procedure. Because of long examination duration, MRI/MRA is often performed under sedation or general anesthesia in young children. Given recent concerns for potential neuroapoptotic effects in the brain of young patients, coaching for nonsedated examinations is encouraged. Additional limitations of MRI include its ability to detect calcifications and osseous changes that may be seen in aggressive lesions. Techniques such as diffusion-weighted imaging and dynamic contrast-enhanced imaging can be used for procedural planning on where to sample the soft tissue lesions, and in the future may be used to determine treatment response and necrosis rates. MR spectroscopy, elastography, and perfusion imaging also provide potential and promise in characterization of soft tissue masses.
Some basic technical components of the examination are relevant. Although a detailed discussion about the use of coils is out of the scope of this chapter, using the smallest possible coil for the question being asked and anatomic region being imaged is important. Likewise, each vendor and MRI machine will have different capabilities, sequence names, and software packages, so a discussion on acronyms would be of limited value. The general key is making sure the basic demographics, clinical and imaging history, reasonable differential, anatomic region for imaging, and the question to be answered are known and then to protocol the most efficient and reliable means for which to answer that question. In addition, one must remember for MR evaluation of soft tissue tumors that are visible and/or palpable that one should always consider use of a surface marker. This becomes even more important with smaller lesions, but one caveat is pressing against a compressible vascular lesion, especially venous, with the marker because this may obscure the findings. In this instance or if any question about obscuring a small superficial lesion exists, one should place a surface marker above and below the lesion.
Articular/Periarticular Mass
The most commonly encountered masses that involve an articular joint are cystic lesions. This includes entities such as ganglion cysts, meniscal cysts, and synovial cysts. They usually present as simple cysts. With these commonalities, they can often be difficult to distinguish from one another.
In the situation where a suspected lesion is atypical with regard to appearance (e.g., heterogeneous signal intensity on imaging), location, or that solid components are unable to be ruled out, gadolinium-based contrast material may be used. With gadolinium-based contrast a true cystic lesion will not show internal enhancement, typically only rim enhancement.
Is the Mass Cystic?
Ganglion cysts, meniscal cysts, and synovial cysts can be difficult to differentiate because of their location and similar imaging appearance on MRI. As will be a common theme, correlating clinical history with imaging is important in narrowing your differential diagnosis. On imaging, a communication with an adjacent joint or association with an effusion of the joint or tendon sheath can help determine the diagnosis. This can many times be done with a linear array transducer and close attention as to the flow direction of any internal echoes during compression, which may give the location of communication and origin.
Ganglion cysts are lesions with a dense fibrous connective tissue capsule lined with flat spindle-shaped cells. Although imaging of these lesions usually shows a simple cyst, septations may be present. Characteristics on imaging that may suggest a ganglion cyst include internal septa with peripheral fluid-filled pseudopodia that shows up as a “bunches of grapes” appearance. Typically, T1-weighted imaging of a ganglion shows intermediate to high signal intensity because of the content being either mucinous or hemorrhagic. T2-weighted sequences will show homogeneous high signal ( Fig. 16.1 ). US will show a similar cystic appearance, and color Doppler imaging may show vascularity of the septa on low-flow settings. Although controversial, ganglion cysts are thought to arise from mucoid cystic degeneration in a collagenous structure under repetitive stress, and hence most commonly occur in the hand, wrist, and foot. The joint capsule and tendon incur the majority of the stress from repetitive activities and results in the periarticular soft tissue being prone to developing ganglia. Ganglion cysts may also occur in intraarticular, intraosseous, or periosteal locations.

Ganglion cysts are less common in children than in adults. When present, these lesions are usually asymptomatic. Management is conservative due to the tendency for ganglion cysts to spontaneously resolve, especially in the pediatric population. Repeated trauma, however, can result in progression of these masses and can lead to them becoming symptomatic or eroding adjacent bone.
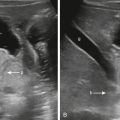
Synovial cysts are commonly confused with ganglion cysts. The lesions primarily differ in that synovial cysts result from herniation of the synovial membrane through the joint capsule or fluid distention of a periarticular bursa and are thus lined by synovial cells. Although synovial cysts may occur in many locations, one of the most well-characterized areas is the popliteal region, where it is known as a Baker cyst. Baker cysts arise from the gastrocnemius-semimembranosus bursa and are specifically located in the medial aspect of the popliteal fossa between the medial head of the gastrocnemius muscle and the semimembranosus tendon. Complications of Baker or popliteal cysts include compression of adjacent structures, rupture, and, rarely, hemorrhage ( Fig. 16.2 ), which have associated changes in the MR signal characteristics but classic anatomic location.

In the pediatric population, imaging of these lesions is unlikely to be associated with a joint effusion or internal derangement. Juvenile idiopathic arthritis is the primary exception to this, where popliteal cysts are shown in the majority of cases and commonly have an associated joint effusion. In children, management is normally conservative with the typical natural history being that of spontaneous involution.
Clinical history of trauma with the presentation of a mass in a joint space may suggest a meniscal cyst. These cysts occur as synovial fluid accumulates within the degenerated tissue from the injury. These cysts develop in continuity with complex meniscal tears, especially if there is horizontal cleavage. These cysts may be contained in the torn menisci as an intrameniscal cyst or may be displaced through the tear. The latter is more common and results in an expansion of the meniscocapsular margin and displacement of the capsule outward into the adjacent tissues becoming a “parameniscal cyst.”
MRI shows meniscal cysts, as well as circumscribed lesions that are often described as septated fluid collections. The continuity with the horizontal cleavage or complex meniscal tear that caused their formation can often be appreciated on imaging as well. Unique features that may be seen with these lesions include low signal intensity contents on T2-weighted imaging. This finding is likely the result of water resorption by parameniscal tissue or hemorrhage. T1-weighted imaging may show these lesions as being isointense to muscle, likely because of hemorrhage or highly proteinaceous content within the cyst.
Synovial venous malformations, most frequently seen in the knee, may appear as an intraarticular cystic mass on an unenhanced MRI examination. Clues to the diagnosis include recurrent hemarthrosis and hemosiderin deposition within the joint. If a synovial venous malformation is suspected, repeat contrast-enhanced MRI can be performed for diagnostic confirmation.
Does the Mass Enhance With a Gadolinium Contrast Agent?
When considering your differential for an articular mass, enhancement with gadolinium contrast can be an important differentiator from a cystic lesion. Benign tumors that may be considered include pigmented villonodular synovitis (PVNS) and giant cell tumor of the tendon sheath (GCTTS). PVNS and GCTTS are classified as benign fibrohistiocytic tumors (although there is no relationship with true histiocytes). Synovial sarcoma is an important entity to keep in mind with gadolinium-enhancing lesions due to its malignant potential.
PVNS is an idiopathic tumor of synovial origin that can occur as a diffuse or intraarticular form. This lesion can be seen in the pediatric population (usually in those older than 10 years), although it is much more prevalent in those around 20 to 50 years old. Patients have swelling and pain that develops over time; an acute presentation is rare. It most often affects the knee joint. In the knee, PVNS most commonly occurs in the Hoffa fat pad, the suprapatellar bursa, and the posteromedian synovial joint recess. Additional areas that are affected by PVNS include the ankle, hip, shoulder, and elbow. Multiple joint involvement has been reported in the pediatric population.
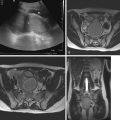
The difficulty in assessing some of these areas with arthroscopy (especially posteromedian synovial joint recess) highlights the importance of assessment with MRI. Findings of PVNS on MRI includes an irregularly thickened or frondlike T1- and T2-hypointense synovium (caused by hemosiderin deposition) ( Fig. 16.3 ). The resulting low signal intensity is exaggerated by signal loss or blooming with gradient-echo or susceptibility-weighted sequences. Despite hypointensity on T2-weighted imaging, PVNS may appear hyperintense on Short Tau Inversion Recovery (STIR) imaging. Avid enhancement after gadolinium is the norm. PVNS may be associated with joint effusions or multiseptated popliteal cyst. Bone involvement (e.g., edema, erosion) is rare in children. Although radiographs may detect any bone involvement and US may detect a large soft tissue mass and reactive effusion, the finer details, anatomy, and characterization of the articular lesions are best delineated with MRI.

Histologically, PVNS is characterized by synovial cell hyperplasia; accumulation of giant cells, macrophages, and fibroblasts; and intracellular and extracellular hemosiderin deposition. Treatment for PVNS is synovectomy. Recurrence for PVNS is estimated to be around 20% to 40%, highlighting the use of MRI in postoperative evaluation as well.
Another entity known as GCTTS is thought to be a continuation of PVNS because of their histological similarities. GCTTS has subclassifications that include a localized extraarticular, localized intraarticular (nodular synovitis), and diffuse extraarticular focus. Localized extraarticular GCTTS is the most common variant. The signal characteristics of GCTTS on MRI are similar to that of PVNS.
Synovial sarcoma, the second common pediatric soft tissue sarcoma after rhabdomyosarcoma, arises from primitive mesenchymal cells (versus synovial, despite the name). It is mostly seen in the first five decades of life. Thirty percent of cases occur in patients younger than 20 years, and it has been reported to occur in children as young as 2 years. Synovial sarcoma usually occurs in the extremities and clinically manifests as a painless, slow-growing mass that has been present for weeks to several years. Other less common clinical presentations include pain and tenderness before the mass becomes palpable, acute arthritis or bursitis, or chronic contracture.
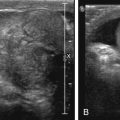
The majority of cases of synovial sarcoma occur in a juxtaarticular location, close to the joint capsule, tendons, and bursa. Only a minority of cases are intraarticular. Plain films findings may include a soft tissue density and spiculated calcifications, seen in up to 30% of cases. With US, synovial sarcoma can appear as a well-defined hypoechoic vascular mass near, but not within, the joint ( Fig. 16.4 ).

A classic MRI description of synovial sarcoma based on the most common findings is a juxtaarticular mass with a heterogeneous or triple-signal pattern (high-, intermediate-, and low-intensity areas) on T2-weighted imaging with fluid–fluid levels secondary to intralesional hemorrhage. Synovial sarcoma tends to be a well-defined mass isointense to muscle on T1-weighted images. Smaller lesions are homogenous, sometimes simulating cysts on unenhanced images. These small lesions, because of their homogenous hyperintense appearance on T2-weighted imaging and their propensity to displace, rather than infiltrate, adjacent structures, have been misdiagnosed as benign entities. Gadolinium is important in the evaluation of these masses. Unlike a cyst, these lesions will enhance.
Synovial sarcoma does often have an intimate relation to adjacent bone. About half of cases will be contiguous with bone, and about 20% will result in adjacent cortical thinning or medullary invasion, serving as a clue to the diagnosis.
Muscle
Despite some of the ability to make a diagnosis for most soft tissue masses, intramuscular masses lack specific diagnostic features on MRI, but clues are important for narrowing the differential diagnosis. This is especially important to keep in mind for the fibroblastic/myofibroblastic tumor group (includes nodular fasciitis, myositis ossificans [MO], fibrous hamartoma of infancy, myofibroma/myofibromatosis, fibromatosis colli, fibroma of tendon sheath, superficial fibromatosis, deep or desmoid-type fibromatosis, infantile fibrosarcoma, and adult fibrosarcoma). Despite the limited use of MRI for diagnosis, there is still a use for defining extent of involvement. Often these entities will enhance with gadolinium contrast.
Are There Calcifications?
When calcification is present, MO is an important mass to consider. It is a localized, self-limiting, reparative lesion of muscle. Pathologically, MO has three components that include a central zone of proliferating fibroblasts, a middle zone of osteoblasts and foci of immature bone, and a peripheral layer of mature bone with trabeculae. MO is associated with a history of trauma. It can also be associated with repetitive trauma, ischemia, and inflammation. It can appear anywhere in the body, but it is usually found in the areas most associated with trauma, such as the anterior compartments of the thigh and upper extremity.
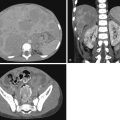
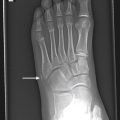
Radiographic and CT findings of MO include serpiginous calcifications 2 to 6 weeks after the traumatic event and a cleft between the calcifications and adjacent bone ( Fig. 16.5 ).

MO follows pathological phases that change with time and correlate with imaging. The acute phase occurs during the first 2 weeks where the lesion may not be visible on T1-weighted imaging or may be poorly defined and with heterogenous signal intensity but predominantly isointense to muscle. On T2-weighted imaging, acute MO is hyperintense. Fluid–fluid levels may also be evident because of hemorrhage. There may be perilesional soft tissues edema and adjacent bone marrow edema.
The subacute phase, from 3 weeks to 6 to 8 weeks, is characterized by peripheral foci of low T1- and T2-signal intensity as a result of bone formation.
Peripheral lesion enhancement and enhancement in the edematous soft tissue may be seen during both acute and subacute phases of MO. This is important to consider in that these imaging findings may mimic an abscess or necrotic tumor.
Many of the aforementioned imaging findings are lost in the chronic stage. During this time (after 6–8 weeks), perilesional edema resolves and MO becomes more well defined. With progression of peripheral ossification, there is resultant more extensive low-signal intensity on all sequences. Overall, regarding MO, unenhanced CT depicts the characteristic peripheral ossification to best advantage.
Calcifications can also be seen in venous malformations in the form of phleboliths ( Fig. 16.6 ). Venous malformations are made up of abnormal postcapillary valveless channels. There is a wide spectrum of lesions within this category, with some lesions affecting a single vessel to some affecting multiple vessels. Venous malformations are congenital but may not appear until later in childhood. Clinically, lesions are soft and compressible and have a bluish hue, but in the setting of acute thrombosis, they may be firm on physical examination, mimicking a neoplasm. When a venous malformation is suspected, the initial diagnostic modality is usually US. Venous malformations can be difficult to detect with US if a lesion is small and the child is large. In addition, it easy for a sonographer to miss a venous malformation if he or she is not careful about pressing too hard, especially with superficial lesions, which can compress the lesion, allowing it to become undetectable. Sonographic findings of a venous malformation include a compressible hypoechoic and sometimes heterogeneous lesion with no flow or monophasic low-velocity Doppler flow. Due to the absence of Doppler flow, venous malformations may be misdiagnosed as a cyst, especially when a fluid–fluid level is seen. Fluid–fluid levels can be seen with very slow-flow venous malformations and is caused by hematocrit levels ( Fig. 16.7 ). Valsalva maneuvers or compression of the affected body part can augment Doppler flow. Venous malformations are prone to thrombosis, and the echogenicity of thrombi depends on their chronicity. Echogenic shadowing foci representing phleboliths are again the best diagnostic clue to this diagnosis.