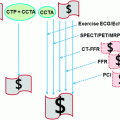
Fig. 1
Scatterplots demonstrating the correlation between quantitative cardiac CTA (QCT) and fractional flow measurements (y-axis) indicating a weak correlation (r = −0.32, right), especially in coronary stenosis >50 % (right). Coronary arteries smaller than 3.5 mm are depicted as solid circles, coronary arteries larger than 3.5 mm as open circles. From Meijboom et al. (2008)
Overall, these observations are not surprising but emphasize the fact that there are two different imaging approaches to CAD, each of which provides fundamentally different information:
(1)
Morphological modalities: Cardiac CTA and invasive coronary angiography, both are well established methods to visualize coronary stenosis and plaque as the anatomic correlate of CAD.
(2)
Functional modalities: nuclear myocardial perfusion imaging (i.e. SPECT), echocardiography, MRI, and invasive measurement of fractional flow reserve are equally reliable in providing information pertaining to CAD.
For the decision of whether invasive or medical treatment is indicated, evidence of functionally relevant ischemia is of critical importance. Thus, in subjects in whom a significant stenosis is detected on CT, in the absence of hemodynamic information, the potential benefits of either revascularization or medical therapy remain undetermined. In order to design evaluation algorithms that allow optimal integration of cardiac CTA, investigations comparing MSCT with other noninvasive functional modalities are mandatory (Schuijf and Bax 2008). This can also be observed in larger randomized trials using cardiac CTA for patients with acute chest pain. For instance, there is interesting data from the ROMICAT II study, a large randomized trial in 1,000 subjects with initially inconclusive evaluation in the emergency department (Hoffmann et al. 2012). While there was an overall benefit from using cardiac CTA in patients with acute chest pain, the benefit was primarily attributed to patients in whom the presence of a significant coronary stenosis was ruled out. In support, subjects who were found to have CAD by CTA were predominantly referred for subsequent functional testing.
From a CT perspective, this need of functional information has resulted in a new field research, which extends the CT-based technology from morphological to functional assessment.
3 Rationale for Functional Imaging
Functional imaging relies on the detection of the hemodynamic consequences of CAD—known as ischemia—rather than pathological changes in the coronary arteries. In the presence of ischemia, defined as the mismatch of oxygen demand and supply, a sequence of events is initiated, referred to as “the ischaemic cascade” (Nesto and Kowalchuk 1987). Perfusion abnormalities start as diastolic and progress toward a systolic dysfunction; only at the very end of the cascade do ECG changes and angina occur (Fig. 2). Accordingly, the occurrence of perfusion abnormalities during stress may be more sensitive for the detection of CAD than the induction of systolic dysfunction (wall motion abnormalities) (Leong-Poi et al. 2002).
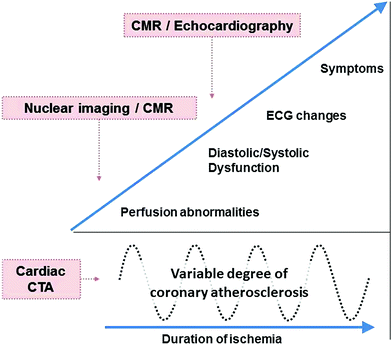

Fig. 2
The ischemic cascade represents the sequence of pathophysiological events following ischemia, with perfusion abnormalities occurring in the beginning, which can be identified by nuclear imaging techniques or CMR. The amount of the underlying degree of coronary atherosclerosis can vary and is detected and characterized by cardiac computed tomography angiography (CTA). Modified from Schuijf et al. (2005) CMR cardiac magnetic resonance imaging
As coronary atherosclerosis often occurs without any hemodynamic consequences, the likelihood that a coronary stenosis may be flow limiting is related to the degree of stenosis and vice versa; severe perfusion defects are highly predictive of a critical (>90 %) coronary stenosis (Shaw and Berman 2009). Notably, a perfusion deficit may also be associated with vascular dysfunction in the setting of non-obstructive CAD, as collateral flow in a patient with obstructive CAD may result in normal flow to that region (Aarnoudse et al. 2003).
It is obvious that CAD is not necessarily a prerequisite or an immediate cause of myocardial ischemia, as there are concordant and discordant findings between functional and morphological imaging modalities. Overall, these observations explain the level of discrepancy observed in CTA studies as detailed above.
4 Established Techniques for Functional Imaging
There are three major functional imaging modalities: myocardial perfusion assessment by SPECT, wall motion abnormalities detection by echocardiography, or both of these through CMR (Marcus et al. 2011). Over time, these techniques have become complementary rather than competitive, since they provide different information. Generally, images are interpreted visually by adhering to a 17 myocardium-segment model, developed for SPECT but usually applied to all functional cardiac imaging modalities (Fig. 3) (Cerqueira et al. 2002).
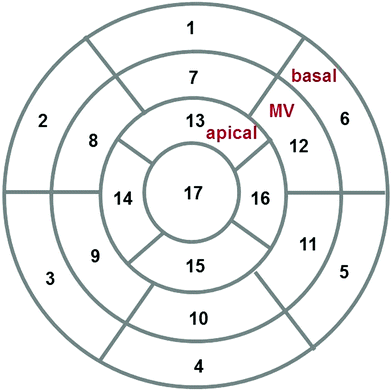

Fig. 3
Seventeen segment model developed for assessment of myocardial perfusion abnormalities, which is used to report findings for all functional imaging modalities. Modified from Cerqueira et al. (2002)
It is important to note that although these functional imaging modalities primarily focus on the detection of perfusion defects as part of the ischemic cascade, usually their diagnostic accuracy is studied and reported for the detection of significant coronary stenosis. This, however, is not the appropriate comparator (as detailed above), given the discrepancy between function and morphologic information.
4.1 Nuclear Myocardial Perfusion Imaging
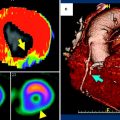
SPECT, using technetium-, thallium-, or tetrofosmin-based radionuclide tracers, has become the most widely used modality for the assessment of myocardial perfusion. By comparing the uptake and distribution of radionuclide by myocytes under rest and stress conditions, it allows a three-dimensional assessment of the myocardial perfusion and viability. Reversible defects (perfusion abnormalities detected at stress but absent at rest) are indicative of ischemia; persistent defects present both in rest and stress condition represent infarcted myocardium. Examples of a reversible and persistent defect are provided in Figs. 4 and 5, respectively.
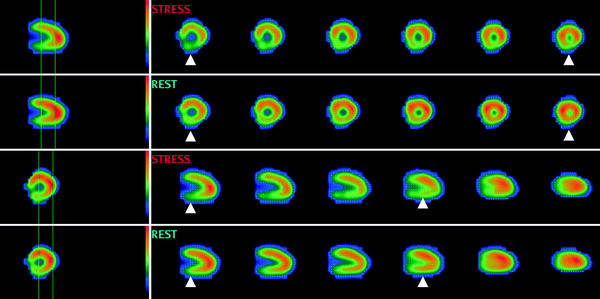
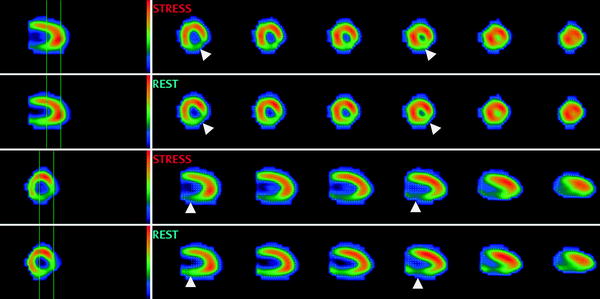

Fig. 4
Myocardial perfusion imaging (tetrofosmin) SPECT study of a 78 year old male with a reversible defect in the posterior wall consistent with ischemia (arrowheads). Courtesy of Dr. S. Lehner, Munich, Germany

Fig. 5
Myocardial perfusion imaging (tetrofosmin) SPECT study of a 58 year old female with a persistent defect in the posterior wall consistent with infarction (arrowheads). Courtesy of Dr. S. Lehner, Munich, Germany
The diagnostic accuracy of SPECT in detecting morphologically significant coronary stenosis is relatively high (specificity: 87–94 % and sensitivity: 85–90 %) (Rochmis and Blackburn 1971; Mertes et al. 1993; Kapur et al. 2002; Taillefer et al. 1997; Mark et al. 2003; Merhige et al. 2007). Disadvantages of SPECT include its tracer-dependent radiation dose (9–25 mSv) and a total examination time of ~4–5 h (imaging time 10–20 min per session, with 3–4 h separation time between stress and rest acquisitions). Associated costs are moderately high (approximately $600–$1,000) (Merhige et al. 2007). However, there is evidence that recently improved technology, i.e. ultrafast cameras/multiple scanning detectors (D-SPECT), may reduce radiation dose, shorten study time, and improve sensitivity (Garcia et al. 2011).
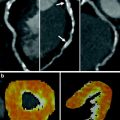
4.2 Cardiac Magnetic Resonance Imaging
In contrast to SPECT, MRI is a non-ionizing radiation modality that is increasingly used for the assessment of cardiac function and myocardial perfusion (Cheong 2010). Contrast material (gadolinium chelate) has a fairly linear relationship between its concentration and signal intensity on T1-weighted sequences (Croisille et al. 2006; Donahue et al. 1997). Besides its merit in the evaluation of non-ischemic cardiomyopathies, MRI permits the differentiation between persistent defects (fixed defects, present both in rest and stress conditions, consistent with infarction) and reversible defects (present on stress acquisitions only, consistent with ischemia). Late gadolinium enhancement (pathological myocardial enhancement 10 min after contrast administration) is the hallmark of infarction; an example is provided in Fig. 6.
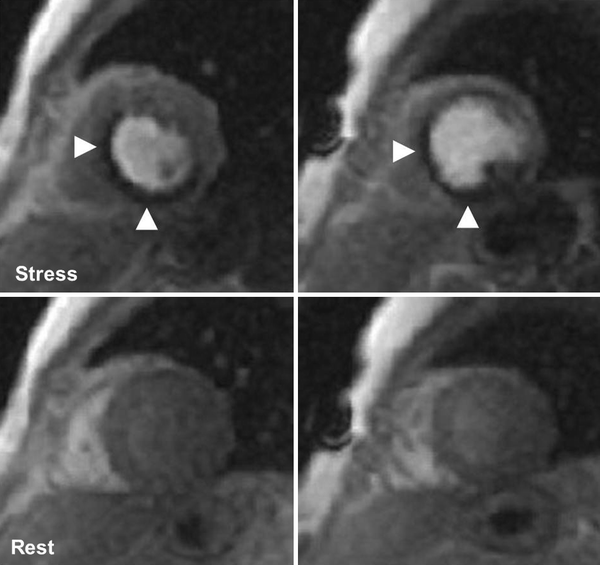

Fig. 6
Cardiac magnetic resonance imaging study (1.5 T) of a 61 year old male with an adenosine-stress induced perfusion defect in the inferior and infero-septal wall (arrowheads)
In contrast to SPECT, MRI allows the visual differentiation between subendocardial and transmural infarction, a very relevant finding for guiding therapeutic intervention. Its diagnostic accuracy for the detection of morphologically significant coronary artery stenosis is high (sensitivity and specificity of 89 and 87 %, respectively) (Christiansen et al. 2010; Klem et al. 2006). There is recent data suggesting that MRI can accurately detect hemodynamically relevant CAD similar or slightly superior to SPECT (Schwitter et al. 2008).
Disadvantages of MRI include the required staff expertise, limited availability in community hospitals, relatively long acquisition times (30–45 min), high cost, and contraindication in patients with pacemakers or implanted defibrillators.
4.3 Echocardiography
Echocardiography, the least expensive technique, is well-suited for the assessment of cardiac function, specifically global and regional dysfunction. Strain rate, which is the systolic shortening of the longitudinal and circumferential axes (negative strain) by a thickening or lengthening in the radial direction (positive strain) can be quantified by either Doppler or 2D ultrasound technique (Marwick 2006). Also, in the diastolic phase, left ventricular impairment (diastolic stunning) is a very sensitive marker of myocardial ischemia (Bonow et al. 1985; Wijns et al. 1986; Ehring and Heusch 1990). The ratio of strain changes before and after exercise has a high sensitivity of 97 % and a specificity of 93 % in detecting a significant coronary stenosis (Ishii et al. 2009; Achenbach et al. 2010), which may be improved by administration of contrast media (microbubbles) (Hundley et al. 1998).
Its main limitations include a very high observer dependency associated with a high interobserver variability.
5 Relevance of Functional Imaging for Patient Management
The clinical value of functional imaging for patients with suspected or known CAD is complex but can be simplified and categorized into a number of scenarios in which obtained functional information have shown to be beneficial for patient management.
5.1 Identification of Treatable Ischemia
Most data demonstrate that perfusion imaging is able to effectively identify subjects for subsequent angiography, with SPECT currently being the most frequently used test in the United States (Min and Berman 2009). Early studies indicate that myocardial perfusion is significantly impaired in the presence of a ≥70 % coronary stenosis (Gould and Lipscomb 1974). SPECT has the ability to identify disease in individual coronary arteries, since perfusion abnormalities correlate closely with coronary artery perfusion territories (Elhendy et al. 2000).
The largest body of evidence of the value of perfusion imaging stems from a cohort of 10,000 subjects without prior CAD, who underwent stress SPECT prior to intervention or medical therapy (Hachamovitch et al. 2003). Despite a non-randomized, retrospective approach, the results indicate that after 2 years of follow-up, subjects with moderate to severe perfusion defects who underwent revascularization had a significantly improved mortality as compared to subjects with no or mild perfusion defects who were revascularized. The value of the assessment of myocardial perfusion imaging has recently been confirmed by the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, the largest randomized trial of patients with chronic CAD (Boden et al. 2007). In the main study, in 2,287 patients, a strategy of optimal medical therapy plus revascularization was not associated with an improved outcome compared with optimal medical therapy alone. The nuclear substudy subsequently published by Shaw et al. confirmed the importance of the quantitative noninvasive assessment of perfusion on serial stress SPECT imaging in a subset of 314 patients (Shaw et al. 2008). Patients having significant improvement in ischemia, which was achieved more often in the interventional group, had fewer subsequent cardiovascular events (Fig. 7).
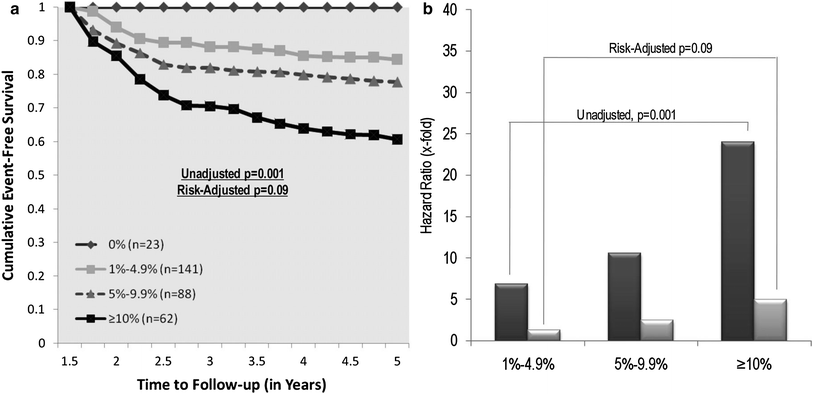

Fig. 7
Kaplan-Maier-survival curves a and hazard ratios associated with residual perfusion defects after treatment b in the COURAGE trial. From Shaw et al. (2008)
Similarly, stress CMR can be used to accurately detect significant coronary stenosis as shown in a recent meta-analysis pooling results of 1,516 patients with an intermediate likelihood of disease (prevalence 57.4 %) (Nandalur et al. 2007). The results by Nadalur et al. indicate a relatively high sensitivity and specificity of 0.91 and 81 %, respectively.
5.2 Relevance of Myocardial Viability
Assessment of myocardial viability is critical for the management of patients with ischemic cardiomyopathy, as only viable myocardium will benefit from revascularization. From an array of imaging modalities, including PET and contrast echocardiography, CMR and SPECT are the predominant methods used for the assessment of myocardial viability, based on perfusion and scan quantification (Schinkel et al. 2007). On CMR, late gadolinium enhancement represents non-viable myocardium or fibrosis (rarely myocarditis), whereas non-enhancing areas represent viable tissue. In SPECT, fixed and reversible defects (between rest and stress acquisitions) differentiate viable from non-viable myocardium, respectively.
If assessed early after MI, there is evidence that the extension of non-viable, infarcted area may predict recovery of left ventricular function. In a small initial study of 40 patients, Choi et al. demonstrated that the extent of dysfunctional myocardium that was not infarcted or had necrosis comprising less than 25 % of the wall thickness, as determined by CMR, was the best predictor of global improvement in the left ventricular function (Choi et al. 2001).
The transmural extension of non-viable, infarcted myocardium predicts the recovery of left ventricular function after revascularization (Kim et al. 2000). Selvanayagam et al. studied 50 patients with known coronary artery disease and left ventricular dysfunction prior to surgical revascularization (CABG) using CMR (Selvanayagam et al. 2004). Following revascularization, subjects were assessed for improvement of left ventricular function. They found that the likelihood of recovery from left ventricular dysfunction decreased with increase of the transmural extent of late gadolinium enhancement (LGE) on CMR. Specifically, they found that 78 % of myocardial segments without necrosis improved, whereas only 17 % of segments with LGE greater than 75 % transmurality improved at follow-up (Fig. 8).
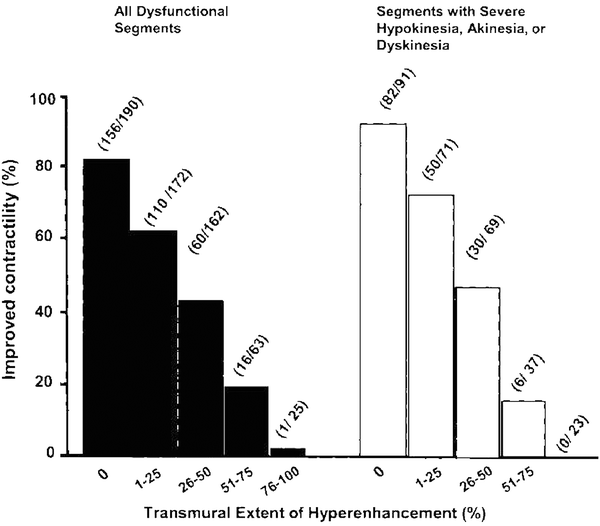

Fig. 8
Relationship between transmural extent of LGE on CMR before surgery and likelihood of increased regional function after surgery in all dysfunctional segments (left) and in all segments with severe hypokinesia, akinesia, or dyskinesia. From Selvanayagam et al. (2004)
While improvement of left ventricular function may be a less relevant outcome, there is also evidence from Allman et al. who demonstrated in a meta-analysis of 24 prognostic studies, including 3,088 patients, that the amount of viable myocardium has strong prognostic implications (Allman et al. 2002). Their results show a low annual death rate in patients who had viable myocardium and were revascularized, whereas the death rate in patients with viable myocardium who were treated medically was substantially higher (3.2 vs. 16 % annual death rate, respectively).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree