Imaging technique
Criteria for viability
Sens
Spec
PPV
NPV
Advantages
Disadvantages
SPECT
Thallium-201
Technetium-99m
Perfusion/redistribution, >50 % peak levels
Perfusion, >50 % peak levels
87
83
54
65
67
74
79
76
Available
Reproducible
Extensive experience
Radiation
Spatial resolution
Long acquisition protocols
FDG-PET
(Perfusion/) Metabolism, >50 % peak activity
92
63
74
87
High accuracy
Quantitative measures
Not widely available
Expensive
Echocardiography
LV wall thickness
Dobutamine
ED wall thickness >6 mm
contractile reservea
94
80
48
78
53
75
93
83
Available
Cheap
Operator dependent
Suboptimal image quality in 20 %
MRI
LV wall thickness
LGE
Dobutamine
ED wall thickness >5.5 mm
<50 % transmural LGE
contractile reserve
96
95
81
38
51
91
71
69
93
85
90
75
Spatial resolution
Tissue characterization
infarct size
Not possible in case of intracardiac devices and claustrophobia
4.1 Nuclear Techniques
4.1.1 Single-photon Emission Computed Tomography
SPECT imaging uses single photon emitting radioisotopes to study the viability of the myocardium. The uptake of the radionuclide perfusion tracers depends on myocardial blood flow and the integrity of the cell membrane. Myocardial segments with maintained radiotracer uptake at rest are viable. However, segments with reduced radiotracer uptake may or may not be viable. In the latter cases, myocardial viability can be assessed by imaging myocardial metabolism or contractile reserve. A strong point of SPECT is the extensive clinical experience as well as the multitude of studies showing the ability of SPECT to predict viability. Also, SPECT imaging is widely available, easy to perform, and highly reproducible. However, due to the limited spatial resolution of SPECT, detection of small non-transmural infarcts is difficult. Additionally, both thallium-201 and technetium-99m studies are subject to attenuation artifacts from the diaphragm and breasts, although this can generally be solved by attenuation corrected SPECT. Finally, a disadvantage compared to MRI and DSE is the associated radiation burden.
4.1.1.1 Thallium-201
Thallium-201, one of the earliest radionuclide tracers, is actively extracted from the blood across the myocyte cell membrane via the sodium potassium-adenosine triphosphate pump. This transport system is unaffected by hypoxia unless irreversible injury is present. Images obtained early after radiotracer injection represent blood flow, whereas retention and redistribution of thallium over a 4–24 h period reflect intact cell membrane function, and thus, viability. Areas of LV dysfunction with thallium-201 activity >50 % of peak levels early after radiotracer injection are considered to be viable. Patients who have segments with < 50 % of peak levels undergo redistribution imaging after 4 or 24 h. Thallium-201 redistributes over time into viable cells independent of the extent of first-pass perfusion. It has been shown that imaging 4 h after stress injection can underestimate the presence of viable myocardium as compared to imaging results at 24 h (Perrone Filardi et al. 1996) and compared to metabolic imaging with FDG-PET (Brunken et al. 1992). Modified protocols that involve reinjection of radiotracer (Bonow et al. 1991; Dilsizian et al. 1990) were found to improve the detection of viable myocardium. Zimmerman et al. (1995) showed that regional thallium-201 activity in redistribution and reinjection images is proportional to the mass of preserved viable myocytes in jeopardized myocardium. Images are often interpreted visually, but relative quantification of regional radiotracer uptake may provide more objective and accurate results (Qureshi et al. 1997). The most common protocols used for viability detection with thallium-201 are: (1) stress-redistribution-reinjection imaging, which provides information about inducible ischemia and cellular viability (Dilsizian et al. 1990); and (2) rest-redistribution imaging, which provides information about myocardial blood flow at rest and cellular integrity (Ragosta et al. 1993). The probability of functional recovery post-revascularization decreases as regional thallium-201 uptake declines. Areas with little or no thallium-201 uptake are unlikely to recover function after revascularization (Perrone Filardi et al. 1996). In a meta-analysis of 40 studies, the weighted mean sensitivity of thallium-201 imaging was 87 %, specificity 54 %, positive predictive value (PPV) 67 %, and negative predictive value (NPV) 79 % (Schinkel et al. 2007). Thallium-201 was found to provide important long-term prognostic information in patients with severe LV dysfunction who underwent CABG (Gurserer et al. 2002). Disadvantages of thallium-201 include a long half-life (73 h) which leads to a relatively high radiation dose, and suboptimal image quality in the cases of obesity and large breasts, which can result in false-positives.
4.1.1.2 Technetium
In recent times, Technetium (Tc)-99m has become the preferred SPECT radiotracer. 99mTc-labeled agents emit higher energy photons than thallium-201, yielding better image quality. Also, the shorter half-life time of 99mTc allows the administration of a higher dose. Flow tracers such as 99mTc-sestamibi and 99mTc-tetrofosmin are lipophilic and positively charged. Unlike thallium-201, Tc-99m-sestamibi and Tc-99m-tetrofosmin are passively transported via the sarcolemmal membrane and bind to the inner membrane of mitochondria (Piwnicka-Worms et al. 1994). Uptake and retention of 99mTc-sestamibi and 99mTc-tetrofosmin is dependent on cell membrane integrity and mitochondrial function (Travin et al. 2005). Regional 99mTc-sestamibi and 99mTc-tetrofosmin activity is closely correlated with the results obtained in thallium-201 imaging (Udelson et al. 1994; Matsunari et al. 1997). The use of nitrates prior to the injection of Tc-99m-labeled tracers enhances collateral flow and thus myocardial uptake in areas of resting hypoperfusion (Aoki et al. 1991). This improves the evaluation of myocardial viability. Another method to enhance the detection of viability is simultaneous assessment of LV function using gated SPECT imaging, which allows assessment of contractility. An example patient is shown in Fig. 1. In combination with low dose dobutamine infusion, gated SPECT imaging can be used to evaluate contractile reserve (Iskandrian et al. 1998). Like in thallium-201 imaging, apart from visual analysis, quantification of tracer uptake can be performed in Tc-99m SPECT imaging. Dysfunctional myocardial segments with >50 % of peak levels are considered viable and have a good probability of functional recovery after revascularization. In contrast, segments showing <50 % tracer uptake at rest have poor viability and a much lower probability of improved function after revascularization. In addition to viability imaging, rest SPECT images can be used for assessment of infarct size (Gibbons et al. 2005). In the previously mentioned meta-analysis, the reported weighted mean sensitivity for technetium-99m SPECT was 83 %, specificity 65 %, PPV 74 %, and NPV 76 % for predicting regional functional improvement after revascularization (Schinkel et al. 2007). Most of the 26 studies in the meta-analysis applied Tc-99m-sestamibi as tracer. Tc-99m SPECT imaging with use of nitrates resulted in better specificity and NPV than without nitrates, with comparable sensitivity and PPV.
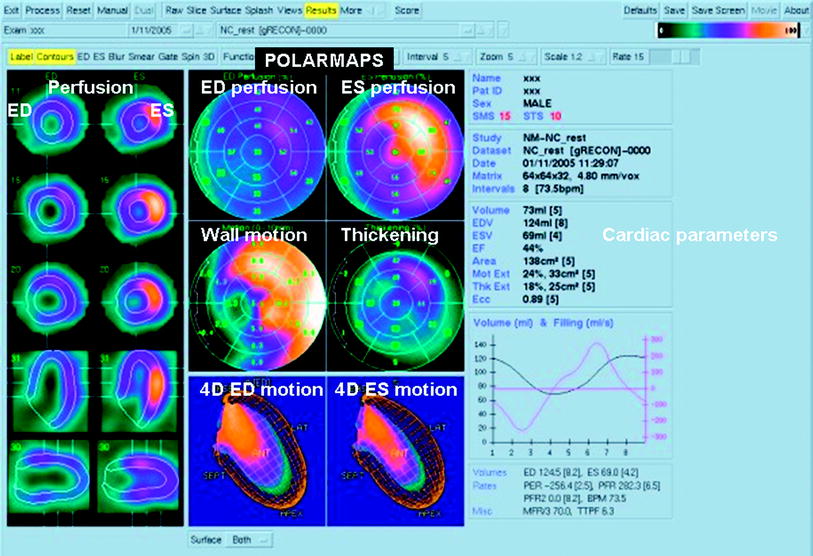

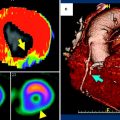
Fig. 1
Gated single-photon emission computed tomography myocardial perfusion scintigram in a patient with a history of inferoseptal infarct. Left perfusion and endo-/epicardial contours, cross-sections of the left ventricle in end-diastole (ED) and end-systole (ES). Middle, above: bull’s eye views of the left ventricle of perfusion in ED and ES, wall motion and thickening. Middle, below: four-dimensional view of wall-motion in ED and ES. Color scale ranges from light yellow (normal perfusion/function) to green (no perfusion/function). On the right side the calculated cardiac parameters, including ED and ES volume and ejection fraction. Courtesy of Dr RHJA Slart, University Medical Center Groningen
4.1.2 Positron Emission Tomography
In PET imaging, radiotracers are used that emit positrons. Upon encountering an electron, the positron annihilates together with the electron, resulting in the production of a pair of 511 keV photons that travel at 180° from each other (Slart et al. 2006). PET imaging consists of detection of these photons when they hit the detectors within a pre-specified time interval (coincidence detection). The radiotracer is then assumed to be positioned directly between the two detectors. A low-resolution CT or a radionuclide transmission image is performed together with PET to correct for attenuation of photons. PET imaging can be applied to assess viability by the measurement of myocardial perfusion and/or metabolism (Fig. 2). Myocardial perfusion is assessed using rubidium-82 or N-13 ammonia. Commonly, myocardial metabolism is assessed by FDG, a glucose analog. The high temporal and spatial resolution of PET (Bacharach et al. 2003) combined with the attenuation correction allows quantification of small amounts of radiotracer uptake and estimation of myocardial blood flow (Al Mallah et al. 2010). Due to the very short half-life of most radiotracers (a few minutes), PET protocols are fast and radiation exposure is lower than for SPECT. FDG has a 2-h half-life that allows transport to sites without an on-site cyclotron. Disadvantages of PET include the high costs of the technology and the limited availability of PET scanners and radiotracers.
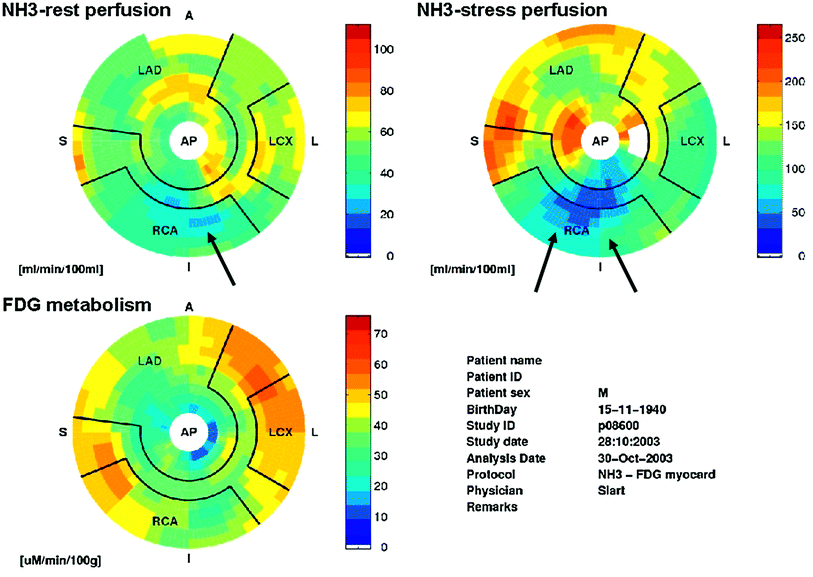

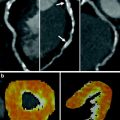
Fig. 2
Positron emission tomography examination. Polarmaps of the left ventricle showing absolute quantification of myocardial perfusion and FDG metabolism. The upper-left polarmap shows rest 13N-ammonia perfusion, the upper right polarmap depicts dipyridamole stress 13N-ammonia perfusion, the lower polarmap shows FDG metabolism. The color scale of the polarmaps ranges from red (good perfusion/viability) to blue (no perfusion/viability). The stress 13N-ammonia polarmap shows a considerable perfusion defect of the inferior left ventricular wall, extending to the basolateral wall (arrows). This defect is largely reversible as shown on the rest 13N-ammonia polarmap (ischemia), with a small persistent perfusion defect (arrow). The persistent perfusion defect shows glucose metabolism on the FDG polarmap, indicating myocardial viability. Courtesy of Dr RHJA Slart, University Medical Center Groningen
The typical FDG PET viability study consists of FDG PET images paired with resting myocardial perfusion images, which can be obtained using SPECT or PET. In viable but dysfunctional myocardium, FDG uptake increases due to a shift to anaerobic metabolism and a preference for glucose rather fatty acid metabolism (Dilsizian et al. 2008). The specific pattern of regional perfusion and metabolism allows classification of myocardium as normal, hibernating, or scar. A myocardial area with a severe perfusion and metabolism defect (termed a flow-metabolism “match”), indicates a transmural or nearly transmural infarct. A territory with a less severe, matched perfusion and metabolism defect represents a non-transmural infarct without viability. Segments with reduced perfusion and normal or increased glucose metabolism (mismatch) indicate jeopardized, viable myocardium. In myocardial areas with repetitive stunning, myocardial perfusion is normal or nearly normal, FDG uptake is normal or reduced, but stress perfusion, if performed, is typically reduced. FDG imaging can theoretically miss viable tissue in regions of thinned myocardium due to partial volume effects (Kuhl et al. 2006).
PET has a high accuracy for the prediction of functional recovery after revascularization (Tillisch et al. 1986; vom Dahl et al. 1994). The accuracy of PET remains high even in patients with the most severe left ventricular dysfunction (LVEF < 25 %) (Marin-Neto et al. 1998). In a meta-analysis of 24 studies (Schinkel et al. 2007), PET had a weighted mean sensitivity of 92 %, specificity of 63 %, PPV of 74 %, and NPV of 87 %. FDG-PET has been considered the reference standard for viability imaging given the extensive clinical experience, the considerable research data, and its relatively high accuracy for predicting functional recovery following revascularization. Exercise and functional capacity have been found to improve to a greater extent in patients with multiple areas of intact viability by FDG-PET, compared to patients with less viable myocardium (Di Carli et al. 1995; Marwick et al. 1992). The extent of perfusion-metabolism PET mismatch, in particular, identifies patients who will have the largest improvement in heart failure symptoms (Di Carli et al. 1995). PET viability imaging can identify patients with LV dysfunction who will derive the most prognostic benefit from revascularization in terms of reduction of cardiovascular events and mortality (Eitzman et al. 1992; Rohatgi et al. 2001). The value of PET was recently assessed in a randomized trial, the PET and Recovery Following Revascularization (PPAR-2) trial. PET-guided management was compared to routine management of patients with ischemic cardiomyopathy (Beanlands et al. 2007b). In the PET arm, recommendations regarding revascularization were based on the amount of viable myocardium. The study found that the composite endpoint of cardiac events/hospitalizations did not occur significantly less in patients randomized into the PET-based approach compared to the routine care arm (30 vs. 36 %). However, in patients who underwent the treatment that was guided by PET results, there was a significant reduction in mortality rate compared with the routine care arm. In a sub-study, the number of viable, ischemic segments (with perfusion-metabolism mismatch) was strongly related to the prognostic benefit of revascularization (D’Egidio et al. 2009). The capability to perform quantitative assessment of perfusion and metabolism is a particular strength of PET.
4.2 Echocardiography
4.2.1 Morphological Assessment
With echocardiography, myocardial viability can be evaluated through measures of LV wall thickness or myocardial contractile reserve (response to dobutamine infusion). The simplest type of viability assessment by echocardiography concerns LV morphology. Patients with a severely dilated LV are unlikely to show functional recovery after revascularization. The higher the LV end-systolic volume, the less likely the LV is to show improvement of contractile function (Bax et al. 2004). For LV volume measurement, three-dimensional echocardiography is more accurate than two-dimensional echocardiography (Lang et al. 2006). Thinned myocardial segments in patients with chronic CAD typically represent non-viable scar. An LV end-diastolic wall thickness >6 mm has been used as a marker to predict functional recovery post-revascularization (Cwajg et al. 2000). Sensitivity was 94 %, specificity—48 %, indicating that patients who will not benefit from revascularization can be identified, but that end-diastolic wall thickness does not predict patients who will recover LV function. In a study by La Canna et al. (2000), patients with referral for CABG underwent echocardiography (morphological and dobutamine stress evaluation) and thallium-201 studies. LV end-diastolic wall thickness >5 mm had higher sensitivity but lower specificity than viability by dobutamine echocardiography or thallium-201 studies. Myocardial segments with LV end-diastolic wall thickness <6 mm very rarely have contractile reserve on dobutamine echocardiography (Schinkel et al. 2002). Thus, an LV end-diastolic wall thickness below 5–6 mm makes contractile recovery after revascularization very unlikely.
4.2.2 Dobutamine Stress Echocardiography
DSE has long been used to assess jeopardized myocardium for viability. Stress echocardiography relies on dynamic assessment of myocardial wall thickening and wall motion during administration of an inotropic agent. The most extensive experience is available with low-dose dobutamine (Pierard et al. 1990; Smart et al. 1993; Watada et al. 1994; Cigarroa et al. 1993; Perrone Filardi et al. 1995; Sicari et al. 2003; Pagano et al. 1998). Dobutamine is a synthetic catecholamine leading to a considerable increase in systolic blood pressure and heart rate, and an increase in myocardial oxygen demand. It has both a positive inotropic and a chronotropic action. The inotropic effect occurs before the chronotropic effect. This positive inotropic effect, which occurs at low doses of dobutamine, is applicable in myocardial viability assessment (Kuijpers et al. 2004; Nagel et al. 1999). DSE is a widely available technique and is relatively easy to implement. However, DSE involves subjective assessment of regional wall motion, which makes the accuracy of the technique operator dependent. Also, suboptimal echo windows limit its use in approximately 20 % of patients.
To assess myocardial contractile reserve, images are obtained at baseline and under increasing doses of dobutamine. Dobutamine infusion typically starts at 5 μg kg body weight−1 min−1 for 3 min, increasing every 3 min to 10, 20, and in some cases, 30, and 40 μcg kg body weight−1 min−1. In case of a low-dose dobutamine stress protocol, 20 μcg kg body weight−1 min−1 is the highest dose used. If ischemia is tested in the same examination, doses up to 40 μcg kg body weight−1 min−1 (high-dose) are infused. At each stage, echocardiographic images are reviewed to identify new wall motion abnormalities and worsening or improvement of pre-existing wall motion abnormalities.
Dysfunctional myocardial segments can present four different responses to dobutamine infusion (Nagueh et al. 1997): (1) progressive worsening of function. This likely represents hibernating myocardium, served by a critically stenosed coronary artery, or a significant scar. In this case there is no contractile reserve, and any increase in energy demand leads to ischemia. (2) No change in LV dysfunction, indicating scar. (3) Sustained improvement in contractility with increasing dobutamine doses; there is likely enough coronary flow even at high oxygen demands, for example in stunned myocardium. (4) A biphasic response in which a segment shows improvement in contractile function at low dose (5–10 μg kg−1 min−1) with worsening at a higher dose (at least 20 μg kg−1 min−1). Hibernating segments showing a biphasic response have contractile reserve, but this reserve is restricted usually due to concurrent coronary stenosis, resulting in ischemia at higher doses. The benefit of proceeding to higher doses of dobutamine, even if contractile reserve is demonstrated at lower doses, is to observe such a biphasic response.
The biphasic response has the best predictive value of the four possible responses to dobutamine in determining improvement in LV function after revascularization. Two studies in this field demonstrated that 72–75 % of dysfunctional segments with a biphasic response showed functional recovery following revascularization (Afridi et al. 1995; Cornell et al. 1998). Functional improvement post-revascularization is less likely in cases of worsening function (9–35 %) or sustained improvement (15–22 %), while recovery is not to be expected in case of no response to dobutamine (4–13 %). High-dose dobutamine protocols have a significantly higher sensitivity and a similar specificity to low-dose dobutamine protocols (Schinkel et al. 2007), and thus, are recommended if there are no contraindications to high-dose dobutamine. In a meta-analysis of 41 studies using DSE to predict improved ventricular function after revascularization (Schinkel et al. 2007), the sensitivity and specificity were 80 % and 78 %, respectively, and the PPV and NPV were 75 % and 83 %, respectively. Only eight of these studies used a high-dose protocol. The high-dose protocols yielded slightly higher sensitivity (83 versus 79 %) and NPV (85 versus 82 %) than the low-dose studies. In the comparison of DSE and nuclear techniques (Schinkel et al. 2007), nuclear imaging modalities had a higher sensitivity for prediction of regional LV functional recovery, while DSE had higher specificity. Nuclear techniques also had higher sensitivity of global contractile function compared to DSE, at similar specificity. In general, DSE has a tendency to underestimate viability while nuclear imaging modalities tend toward the overestimation of viability. A substantial number of non-viable segments by DSE will be interpreted as viable by nuclear imaging (Panza et al. 1995; Cornel et al. 1999). In the presence of significant myocardial viability on DSE, patients who undergo revascularization were found to have a much more favorable prognosis than those treated medically (Afridi et al. 1998; Chaudhry et al. 1999). Conversely, patients with mostly non-viable myocardium on DSE did not derive prognostic benefit from revascularization.
4.3 Magnetic Resonance Imaging
4.3.1 Imaging Findings in Acute and Chronic Situations
Viability imaging with MRI revolves around two approaches: morphology and function. In acute situations, early after primary percutaneous coronary intervention (PCI), function loss and edema are more pronounced; while in the chronic situation, more structural changes occur, ultimately also leading to function loss. Different indices play a role in the assessment of prognosis after myocardial infarction, such as infarct size, right ventricular involvement, papillary muscle involvement, pericarditis, and microvascular obstruction.
T2-weighted imaging (short-tau inversion recovery—STIR-imaging) can be used to assess the amount of edema, visible as high signal intensity. Edema is a sign of acute injury that becomes less pronounced in time. T2-weighted imaging, especially so-called T2* imaging, can also be used to detect hemorrhage in an area of high signal intensity due to edema. Intramyocardial hemorrhage is associated with more severe infarct-related injury (Kumar et al. 2011).
Another helpful technique is first-pass perfusion imaging. In acute or chronic MI, perfusion images can be normal. This implies that perfusion status has recovered, which is a favorable prognostic sign. Prognosis is worse when perfusion imaging is abnormal, specifically with an area of reduced myocardial enhancement after the injection of an intravenous contrast agent (see Fig. 3). This can imply that there is microvascular obstruction (MVO), also called the no-reflow phenomenon. Multiple factors have been suggested that play a role in the no-reflow phenomenon, including microvascular spasm, endothelial dysfunction, inflammation, edema, embolization of thrombus, and plaque (Krug et al. 1996; Kloner et al. 1974). To a certain extent, MVO can also be PCI-procedure related. Taylor et al. (2006) described that elective PCI immediately impaired resting function as assessed with cardiac MRI. Because first-pass perfusion imaging is a dynamic technique involving single-shot acquisition frames, the technique has relatively low signal- and contrast-to-noise ratios. A recent study showed that MVO is actually best detectable on delayed contrast enhancement MRI due to better contrast-to-noise ratio (see Fig. 3) (Nijveldt et al. 2009). However, the absence of a non-enhancing core on late enhancement images does not exclude the presence of MVO, as there is gradual filling-in of the MVO area with contrast in the minutes following contrast injection.
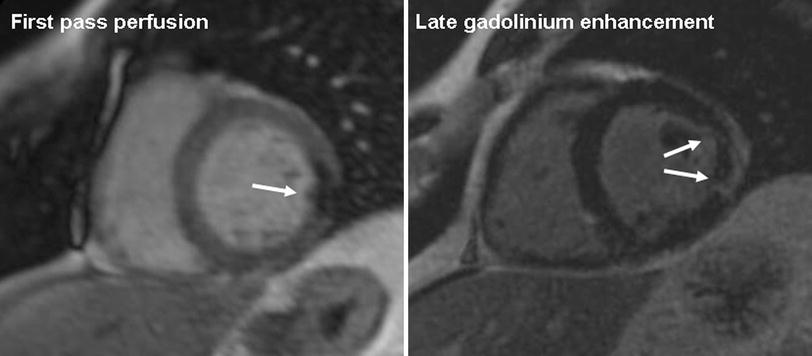

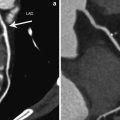
Fig. 3
Magnetic resonance imaging examination in a patient with a partly reperfused infarction, 1 day after the acute coronary syndrome. Midventricular short-axis images. The left image shows a focal perfusion defect during first pass of contrast in the lateral wall, indicative of impaired perfusion and microvascular obstruction. The right image shows late gadolinium enhancement of this area, reflective of necrosis and edema, with a central hypointense area that represents microvascular obstruction. On invasive coronary angiography (not shown), patient had an occluded obtuse marginal branch of the left circumflex coronary artery that could not be reperfused
Functional cine imaging can be used to assess areas of hypokinesia, akinesia, or even dyskinesia as an expression of ischemic damage. In the setting of chronic MI, wall thinning can occur (Fig. 4). In the literature, an end-diastolic wall thickness of more than 5.5 or 6 mm is mentioned as the cut-off for myocardium that recovers function after revascularization (Romero et al. 2012). In a study by Stork et al. (2007), edema on T2-weighted images and wall thinning were accurate measures for differentiating acute from chronic MI, respectively. Delayed contrast enhancement and MVO did not play a role. On the other hand, T2-weighted imaging can substantially underestimate the extent of infarct in the presence of MVO.
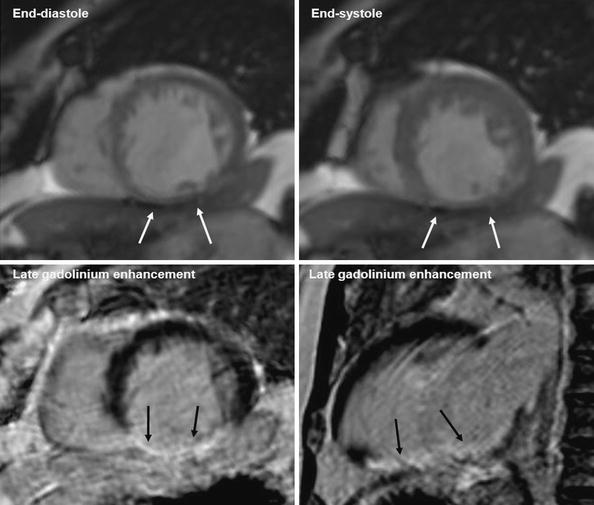

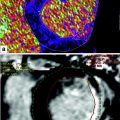
Fig. 4
Magnetic resonance imaging examination. Patient with history of myocardial infarction in right coronary artery territory. Upper row shows midventricular short axis cine slice images in end-diastole (left) and end-systole (right). Left ventricle dysfunction. Thinning of the inferior wall (<5.5 mm), without thickening/contractility in systole. Vertical long axis (left) and short axis (right) lower images show transmural late gadolinium enhancement in the inferior wall. Conclusion: Transmural infarction of the inferior wall without viable myocardium
4.3.2 Late Gadolinium Enhancement
One way to assess myocardial viability by cardiac MRI is the evaluation of late gadolinium enhancement (LGE). In the LGE technique, a T1-weighted imaging sequence is performed 8–10 min after the administration of the contrast agent, Gadolinium. Static imaging is performed, with more signal averaging and thus a higher signal-to-noise ratio than first-pass perfusion imaging. The signal from the myocardium is “nulled”, using an inversion recovery pulse. This results in normal myocardium appearing dark; areas with LGE will then appear relatively bright. The nulling ensures optimal visual contrast between normal and abnormal myocardium. The optimal inversion time for nulling of the normal myocardium differs per patient and sometimes has to be optimized during the acquisition of multiple slices.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree