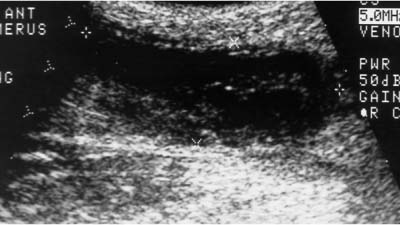
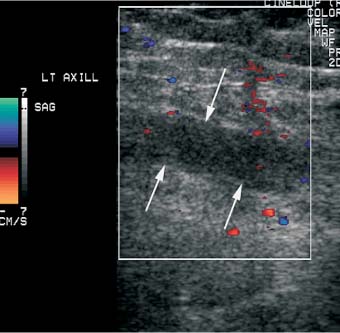
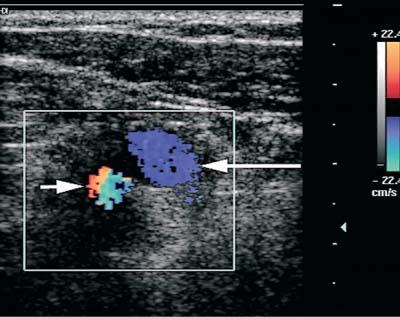
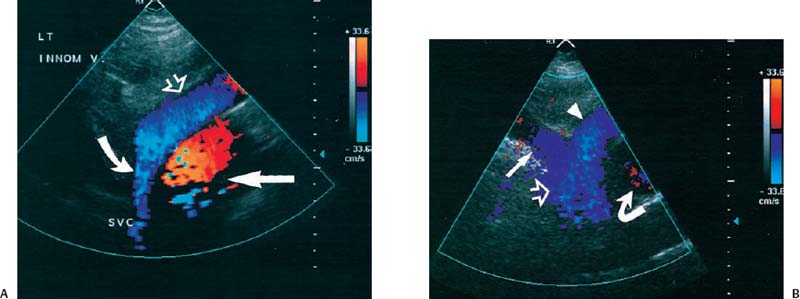
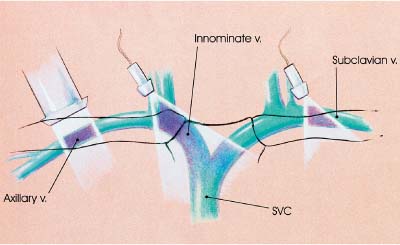
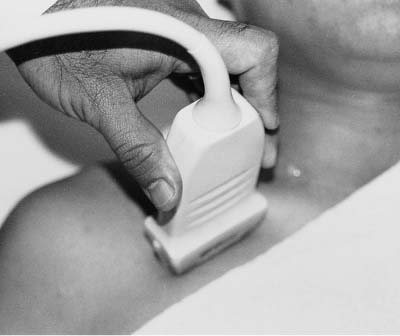
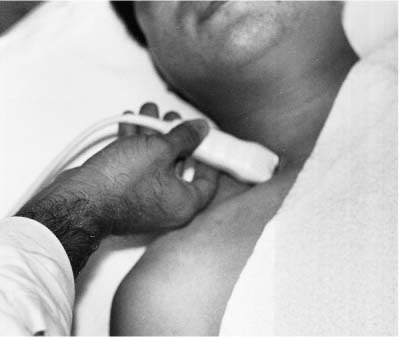
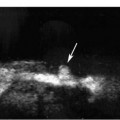
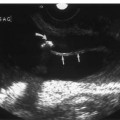
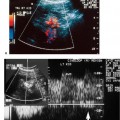
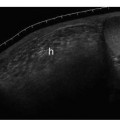
5 Arm Swelling In the upper extremity, several mechanisms can produce swelling, a general term used to indicate the accumulation of extracellular fluids. Increased capillary permeability, decreased oncotic pressure, increased intracapillary hydrostatic pressure, and increased lymphatic pressure are basic mechanisms that lead to swelling. A complete history and physical examination should help identify generalized edema, which is commonly caused by congestive heart failure, renal insufficiency, or nephrotic syndrome.1 Swelling isolated to the arm implies a localized process, such as venous stasis, stenosis, or thrombosis (occlusive or nonocclusive), lymphatic obstruction, cellulitis, or angio-neurotic edema. The patient’s history will often provide some clues to the most likely underlying causes of arm swelling.2 Cellulitis is often caused by a penetrating injury or by the use of nonsterilized needles by intravenous drug abusers. The swelling of cellulitis is often localized, but it can involve the entire arm. If a focal area of the arm is particularly swollen, then an abscess should be considered, and ultrasound can be used to determine whether a fluid collection is present (Fig. 5–1). These collections can be aspirated, diagnostically or therapeutically, and drained under ultrasound guidance. It is important to note that intravenous drug abuse can lead to thrombophlebitis, which can produce swelling and can also coexist with cellulitis. Treatment of breast cancer with axillary dissection and radiation therapy causes arm swelling in as many as 25% of patients; in many of these patients arm swelling develops several years after surgery and radiation therapy.3 The pathophysiology of swelling in these patients is controversial. In 1938, Veal4 stated that 90% of instances of postmastectomy arm swelling were due to venous obstruction. Subsequently, however, Lobb and Harkins5 showed that axillary vein resection did not increase the incidence of arm swelling. Lymphangiographic studies have shown anatomical abnormalities of the lymphatic channels after surgery and radiotherapy,6 but these abnormalities were also seen in patients without arm swelling.7 However, by using color and duplex Doppler sonography, Svensson et al8 showed that 57% of women with arm swelling after breast cancer treatment had evidence of venous outflow obstruction. Venous thrombosis has been shown to occur after radiation therapy alone.9 Thus, venous thrombosis, as well as lymphatic obstruction, should be considered as a possible cause of arm swelling in these patients (Fig. 5–2), particularly because, as will be described, venous thrombosis can lead to significant complications: venous thrombosis is often directly treatable, whereas lymphatic obstruction is not. Other causes of lymphatic obstruction would include trauma or diseases such as elephantiasis, which is more common in the legs and is rarely seen in the Western world.1 Figure 5–1 Single gray-scale ultrasound image of upper arm of patient with cellulitis and history of intravenous drug abuse shows a cystic structure that contains debris and septations. Purulent material was aspirated, confirming the diagnosis of abscess. Figure 5–2 Color Doppler flow image demonstrates low-level echogenic material filling the lumen of the left axillary vein (arrows) of a patient who had undergone left axillary dissection for breast carcinoma. Image shows that no flow is present within the vein. Note that pulse repetition frequency has been lowered to detect any slow flow that may be present. Another cause of extremity swelling, angioneurotic edema, is usually well demarcated and localized. It involves only the deep layers of the skin and adjacent sub-cutaneous tissue. During physical examination, it can be distinguished from other causes of swelling because it is nonpitting; venous stasis and lymphatic obstruction tend to result in pitting edema.10 Angioedema is frequently caused by an allergic reaction and is often self-limiting. Venous stasis in the upper extremity is almost always caused by a venous outflow obstruction that results from partial or complete thrombosis of the axillary, subclavian, or innominate veins, or superior vena cava (SVC); underlying venous stenosis may also be a factor. Unlike the situation in the legs, this form of venous stasis is more likely to result from a central venous process than a peripheral, arm vein pathology. Venous outflow obstruction can also be precipitated by extrinsic compression from an adjacent node, mass, or hypertrophied muscle; such extrinsic conditions can also lead to venous thrombosis, one example being effort thrombosis (Paget-Schroetter syndrome),11 caused by external compression of the subclavian vein usually by hypertrophied scalene muscles; prompt diagnosis of this condition with rapid initiation of thrombolytic treatment followed by surgical correction is recommended to prevent the rather consistent complication of chromic fibrous obliteration of the subclavian vein.12 Current or previous placement of a central venous catheter or peripherally inserted central catheter (PICC) or pacemaker wire through the subclavian vein or internal jugular vein (IJV) and underlying malignancy are the most common predisposing factors for thrombosis; there is some evidence to suggest that the incidence of catheter-related, upper extremity deep vein thrombosis in cancer patients is dependent in part on the location of the catheter tip in the brachiocephalic venous system.13 However, some investigators also believe that a hypercoagulable state is an underrecognized risk factor for developing upper extremity deep vein thrombosis.14–20 Moreover, there is increasing evidence to support that upper extremity deep vein thrombosis is associated with significant risk of pulmonary embolism and a high rate of 1 month and 3 month mortality.16 Because the incidence of thrombosis in patients with indwelling catheters is so high,21 the diagnosis of venous thrombosis should be ruled out in any of these patients who develop arm swelling. Bilateral findings raise the possibility of an obstruction of the SVC caused by either luminal caval stenosis or extrinsic compression from an adjacent tumor, such as lung carcinoma or lymphoma. Arm swelling is only one of several symptoms that can occur as a result of venous outflow obstruction. Other signs include a poorly functioning catheter; local hyper-emia; fever; pain; elevated white blood cell count; appearance of superficial varicosities in the arm, neck, and chest; and facial and neck swelling. However, these clinical signs and symptoms (including arm swelling) are nonspecific. The sensitivity of clinical diagnosis is also very low; thrombosis has been shown to be asymptomatic in 50% of patients with indwelling catheters.22 Prompt diagnosis is important because thrombosis can lead to permanent venous insufficiency, thrombophlebitis, and pulmonary embolism. Venous thrombosis of the upper extremity is associated with pulmonary embolism in up to 16% of cases.17,23,24 History and physical examination are important in determining the cause of arm swelling. Diagnostic tests are used to identify or exclude venous outflow obstruction because it is potentially treatable and can lead to complications. Imaging procedures are required to identify venous obstruction; noninvasive imaging studies represent the most attractive diagnostic alternatives, with venography increasingly limited to the context of minimally invasive treatments.25–27 Contrast venography has been the gold-standard for making this determination. However, venography is an invasive procedure that can cause pain, a phlebitic reaction, or skin necrosis, particularly in patients with thrombosis.28 In addition, the potential for allergic or nephrotoxic reactions exists, and the procedure may be contraindicated in patients with renal failure. Contrast material–enhanced computed tomography (CT) of the chest is accurate for detection of intrinsic and extrinsic obstruction of the SVC. Although accuracy has not been documented, subclavian venous obstruction can be identified using sophisticated CT technologies.29,30 However, CT has the same drawbacks as venography because ipsilateral venous contrast material injection is needed to identify thrombosis, and high volumes of contrast medium are often required for diagnostic studies. Radionuclide venography has been used to assess patency of the upper extremity veins and indwelling catheters, but is used less commonly. False-positive and false-negative results can result from poor venous access, indwelling catheter, or collateral veins being mistaken for the subclavian vein.31,32 Advantages of nuclear imaging are its low cost, relative noninvasiveness, and ability to assess catheter patency. All of these imaging techniques focus on the subclavian vein, but are inherently unable to evaluate the IJV. Kroger et al33 demonstrated that there is a high incidence of IJV thrombosis in patients with subclavian vein thrombosis. Extensive thrombosis of the IJV can lead to dural sinus thrombosis and possible cerebral venous infarction. Magnetic resonance (MR) angiography is capable of imaging the IJV as well as the subclavian vein, innominate vein, and SVC. Two-dimensional time-of-flight MR angiography is accurate and can image the extent of thrombus more completely than venography.34,35 MR angiography has also been used to evaluate occlusion of the subclavian vein in patients with thoracic outlet syndrome.36 Figure 5–3 Anatomical drawing of the upper extremity and thoracic inlet veins shows normal relationships of the internal jugular, innominate, subclavian, axillary, brachial, and cephalic veins. Figure 5–4 Color Doppler image obtained with a linear transducer in a plane axial to the vessel lumina. Note the normal relationship of the axillary vein (long arrow) anterior to the axillary artery (short arrow). Color pulse repetition frequency is set low to visualize flow in the vein. As a result, higher velocity in the artery causes aliasing. Ultrasound is an attractive alternative to other imaging modalities because it is noninvasive, portable, inexpensive, and capable of fully evaluating the IJV, as well as assessing the peripheral veins to establish extent of the process and to provide information on peripheral venous access to the interventional radiologist. However, the test can be challenging to perform and interpret. Sonography of the thoracic inlet veins requires knowledge of the regional anatomy. The basilic and brachial veins merge at the lateral margin of the pectoralis minor muscle to form the axillary vein, which lies medial and inferior to the axillary artery (Fig. 5–3).37 As it courses medially past the lateral margin of the first rib, the axillary vein becomes the subclavian vein, which lies anterior and inferior to the subclavian artery. If a vein becomes occluded, collateral veins enlarge and are often oriented parallel to the subclavian and axillary veins. Part of the sonographic examination involves verifying the close anatomical relationship between the subclavian artery and vein by scanning in a plane perpendicular to the course of the vessels to ensure that a large collateral vessel is not misidentified as the subclavian or axillary (Fig. 5–4). The subclavian vein courses over the first rib and posterior to the clavicle before turning inferiorly and merging with the IJV to form the innominate vein. The right in-nominate vein is shorter and more vertically oriented than the left innominate vein, and the junction of these veins behind the sternal manubrium forms the SVC. In dehydrated patients, visualization of the thoracic inlet veins may be difficult due to lack of venous distention. This situation can be remedied by placing the patient in the supine position with the head flat on the examining table, and elevating the legs if necessary. A complete ultrasound examination of the upper extremity and the thoracic inlet veins includes bilateral evaluation of the IJVs, innominate veins, subclavian veins, and axillary veins. The cephalad portion of the SVC can be visualized in some patients (Fig. 5–5). If thrombosis of the axillary vein is present, the distal extent of thrombus can be documented by examining the brachial vein and its tributaries. Unless the patient is an IV drug abuser, has had trauma or surgery in the arm, or has a dialysis fistula, isolated thrombosis of an arm vein is rare. In addition, unless a dialysis fistula is present, isolated thrombosis of an arm vein is usually not symptomatic and is of less importance than a more proximal thrombosis because of the presence of a large number of potential collateral veins; however, in the face of thrombosis in the context of peripheral dialysis access, central subclavian stenosis should be considered. Gray-scale ultrasound, color Doppler imaging, and pulsed Doppler imaging are all essential for sonographic evaluation of the upper extremity veins.38 A high frequency (7 to 12 MHz) linear transducer is usually optimal for visualizing and compressing the peripherally located portions of the subclavian veins and IJVs, as well as the axillary vein and veins of the upper arm (Fig. 5–6, Fig. 5–7). Overlying osseous structures render visualization of the more centrally located veins more difficult. Figure 5–5 Color Doppler images of the central thoracic veins in a normal patient. (A) Coronal image obtained through the left supraclavicular fossa with a phased-array 7 to 4 MHz transducer shows the central portion of the left innominate vein (open arrow) turning into the superior vena cava (SVC) (curved arrow) over the aortic arch (arrow). (B) Coronal image with the transducer angled more to the right and slightly twisted to visualize the SVC bifurcation. The right innominate vein (arrow) joins the left innominate vein (arrowhead) to form the SVC (open arrow). The edge of the aortic arch (curved arrow) is also visualized. Because of the greater ease with which the sonographer can angle the scan plane, a small footprint phased-array or sector transducer (4 to 8 MHz) aids in visualization of the inferior IJV, the medial half of the subclavian vein, the in-nominate vein, and the SVC. Proper positioning of the probe is essential for imaging these vessels. The junction of the subclavian and jugular veins is best visualized with the probe positioned in the supraclavicular fossa, angling medially, inferiorly, and slightly anteriorly (Fig. 5–8, Fig. 5–9). Note that the veins in this region are located anterior to the arteries. A small footprint 8 MHz transducer can be used in thin patients to view the superior portion of the innomi-nate veins. A 4 MHz small footprint transducer is sometimes required in large patients and for evaluating the lower innominate veins and SVC (Fig. 5–5). Figure 5–6 Drawing demonstrates probe positioning for sono-graphic evaluation of the thoracic inlet veins. The linear transducer can be used peripherally in the axillary vein and internal jugular vein. A small-footprint phased-array transducer is placed in the supraclavicular notch and angled medially to visualize the innominate veins and superior vena cava (SVC). The probe is angled more laterally to visualize the remaining portion of the subclavian vein. Figure 5–7 A 7 MHz linear transducer is used to visualize the axillary vein, inferior to the clavicle. The transducer should be turned 90 degrees to perform compression and confirm a normal relationship of the axillary vein to the axillary artery, to avoid mistaking a collateral vein for the axillary vein. Figure 5–8 Positioning of small-footprint probe to visualize the right innominate vein and superior vena cava (SVC). Anterior view shows that the probe should be positioned in the supraclavicular fossa and angled medially. On the left side, greater medial angulation may be required, and, in some patients, sliding the probe toward the suprasternal notch may allow better visualization of the SVC (see Fig. 5–5).
Differential Diagnosis
Diagnostic Evaluation
Ultrasound Imaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree