Author
Therapy
No. of tumors
Diameter (cm)
Volume of ascites (ml)
TER (%)
LTP (%)
Follow-up (months)
Complication (%)
Nishimura et al. [12]
RFA
46
2.0
500–2,000
100
4.5–5.7
12
0
Song et al. [2]
RFA
148
2.2
436 ± 273
85.3
12
20.4
2.1
Hirooka et al. [7]
RFA
44
2.5
N/A
100
0
12
0
17.5
24
Kondo et al. [14]
RFA
58
2.3
681 (mean) (250–3,000)
96
4
11.2
1.9
Liu et al. [26]
MWA and RFA
34
3.1
150–1,000
90.4
5.8
N/A
7.3
Our research
MWA
36
2.8
633 ± 359 (100–1,500)
96.9
16.1
12.1
2.8
11.3 Introduction of Artificial Ascites
The introduction of artificial ascites can be achieved by various techniques with the use of different equipment such as angiosheath, spinal needle, and intravenous catheter. Although the successful induction rate and the puncture time among the use of these techniques are not significantly different, we prefer the technique using the angiosheath or intravenous catheter because of their soft sheathes or catheters which can sustain saline infusion during MWA without real-time monitoring of the tip of the sheathes or catheters on ultrasound. Normal saline (0.9 %) and 5 % dextrose solution can be utilized as a protective fluid [2, 15]. Infusion of 0.9 % saline solution into the abdomen is safe and effective during MWA procedure in our study.
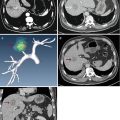
Puncture of a catheter is performed under local anesthesia. Following the administration of local anesthesia with 1 % lidocaine to the skin, abdominal wall, and peritoneum, an intravenous catheter is inserted into peritoneal cavity under ultrasound guidance. When ultrasound shows that the catheter is inserted into the correct site along the edge of the liver, the outer catheter is advanced further to position the tip near the hepatic tumor whenever possible, and the inner stylet is removed (Fig. 11.1a, b). Once the catheter is in place, a sufficient amount of 0.9 % saline solution is injected until a separation of at least 5 mm between the index tumor and the adjacent GT is achieved (Fig. 11.1c). After the successful induction of artificial ascites, the MWA procedure is performed (Figs. 11.1d and 11.3a, b). Sometimes it is very difficult to insert the catheter directly into the correct site due to the GT’s interference. The method of puncture step by step is useful (Fig. 11.2). The intravenous catheter is inserted into the shallow site firstly, and then a small amount of saline is injected to separate the GT or omentum from the liver surface. Meanwhile, the tip of the catheter could be well revealed. Next, the catheter is inserted deeper, and the process is repeated until the tip of the catheter is positioned near the index tumor.




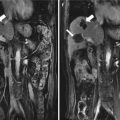
Fig. 11.1
Artificial ascites technique in assisting microwave ablation (MWA) of hepatic tumors adjacent to the gastrointestinal tract (GT). (a) An intravenous catheter (arrow) is inserted into the space between the surface of the liver and the GT along the edge of the liver under ultrasound (US) guidance. (b) The outer catheter (arrow) is advanced further to close to the index tumor whenever possible; next, the inner stylet (arrowhead) is removed. (c) The saline solution is injected via the catheter (arrow) until the GT is separated successfully from the index tumor. (d) After the successful introduction of artificial ascites, the MWA procedure (arrow) is performed safely, avoiding the thermal injuries of the GT (☆). Note the microwave antenna (arrowhead)

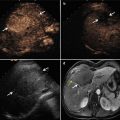
Fig. 11.2
The method of puncture step by step. (a–e) An intravenous catheter (arrowheads) is inserted into the shallow site firstly. (b, f) A small amount of saline is injected to separate the shallow GT (☆) from the index tumor (arrow). (c, g) The catheter (arrowheads) is inserted deeper and the saline is injected sequentially. (d, h) The process is repeated until the deep GT (☆) is separated successfully from the index tumor (arrow)

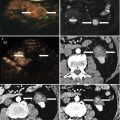
Fig. 11.3
The use of thermal monitoring system and contrast-enhanced US during MWA assisted by artificial ascites. (a, b) The thermocouple (arrow) is placed into the artificial ascites. Note the catheter (arrowhead). (c, d) Contrast-enhanced US is helpful to well display the position of the catheter by observing the contrast agent’s diffusion (arrowheads)
Contrast-enhanced ultrasound can be performed to aid the good revelation of the catheter position if conventional ultrasound failed to visualize it clearly. During the insertion of catheter, a minute amount (0.1 mL contrast agent dissolved in 10 mL 0.9 % saline) of ultrasound contrast agent (SonoVue, Bracco, Milan, Italy) is injected into the catheter, and the position of catheter could be revealed clearly by observing the contrast agent’s diffusion (Fig. 11.3c, d).
11.4 Efficacy of Artificial Ascites in Assisting MWA for Hepatic Tumors Adjacent to the GT
Kondo et al. [14] firstly reported that the method of artificial ascites made RFA safe and effective for hepatic tumors abutting the GT. Later, several experimental and clinical researches have been reported, which verified the safety and effectiveness of the treatment of hepatic tumors abutting the GT using RFA assisted by artificial ascites. An in vivo experiment suggested that artificial ascites during RFA did not cause heat-sink effect that could affect the volume of ablation zone [16]. A clinical study reported by Song et al. [2] included 143 patients with 148 hepatocellular carcinoma nodules abutting the diaphragm and the GT treated by RFA procedure with artificial ascites. The study showed that artificial ascites was a simple and effective method to avoid thermal injury and improve tumor visibility, with an artificial ascites induction success rate of 90.9 %, primary technique effectiveness of 85.3 %, and local tumor progression of 12 %.
MWA has gained increasing attention due to its higher intratumorous temperature, larger ablation volume, shorter ablation time, and lesser heat-sink effect compared with RFA [17–20]. And multicenter studies have validated that the effectiveness and safety of MWA in the treatment of hepatic tumors [6, 21]. However, few studies which access safety and effectiveness of MWA assisted by artificial ascites for the treatment of hepatic tumors abutting the GT have been reported. Liu et al. reported the value of thermal ablation (including RFA and MWA) with the use of artificial pleural effusion or ascites for treating 56 hepatic tumors close to the diaphragm or the GT. The separation success rate of artificial ascites for 34 tumors close to the GT was 91.2 % (31/34), and the first-time complete ablation rate in 52 cases with successful artificial pleural effusion and ascites use was 90.4 % (47/52), but there were no detailed data about the two thermal ablation modalities in the report.
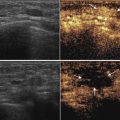
In our latest study, a total of 36 patients with 36 hepatic malignancies that were located <5 mm from the GT underwent the introduction of artificial ascites before ultrasound-guided percutaneous MWA. These patients included 25 cases with hepatocellular carcinoma and 11 cases with metastatic liver cancer. The mean tumor size was 2.8 ± 1.0 cm. The separation success rate of artificial ascites was 88.9 % (32/36), and the technical effectiveness of MWA in 32 cases with successful separation was 96.9 % (31/32) with a short ablation time (423 ± 124 s) (Figs. 11.4 and 11.5). A thermal monitoring system attached to the microwave unit was used to continuously measure temperature in real time during the MWA procedure. One or two thermocouples (Kangyou Medical, Nanjing, China) were placed into the hepatic tissue around the tumor margin proximal to the GT or the artificial ascites between the index tumor and the GT under ultrasound guidance (Fig. 11.3a, b). Once the measured temperature reached 54 °C, the emission of MWs was stopped immediately and then reactivated after the temperature decreased to 45 °C. The study data showed the temperature of artificial ascites was significantly lower than that of tumor margin (39.1 ± 4.6 °C vs. 52.3 ± 4.5 °C, P < 0.001). The temperature data verifies that artificial ascites play a role of cooling and preventing thermal injury to the adjacent GT, even with higher temperature and faster temperature rising during MWA process. The cooling effect did not cause heat-sink effect to reduce the efficacy of MWA, as shown by the high technical effectiveness of MWA in our study.