Ultrasound Assessment of the Liver Transplant Candidate
Introduction
Ultrasonography is frequently the initial investigation in patients with hepatobiliary disease, and the same is true of the evaluation of candidates for transplantation, with its advantages of being readily available, relatively inexpensive, and involving no ionizing radiation. The majority of patients referred for assessment have chronic liver disease, the cause of which has already been established. Therefore, the emphasis in investigating these patients is on recognizing the sequelae of chronic disease and excluding any contraindications to transplantation.
A smaller, but significant, population will present with acute liver failure having had no antecedent hepatic dysfunction. The role of the radiologist in this group is more limited, but will be considered later in the chapter. Finally, the role of ultrasound within the neonatal and pediatric transplant assessment will be reviewed, as this group has a different spectrum of disease and requires the use of novel surgical techniques.
Chronic Liver Disease
Parenchymal Imaging
The sonographic techniques used in imaging the liver are well described.1 In imaging the patient with chronic liver disease, they remain essentially unchanged, but may require the use of a lower-frequency transducer (2-3 MHz) than usual in order to compensate for both the increased attenuation seen in the cirrhotic liver and for the presence of ascites. This will, of course, reduce the resolution of the image, so a balance should be found.
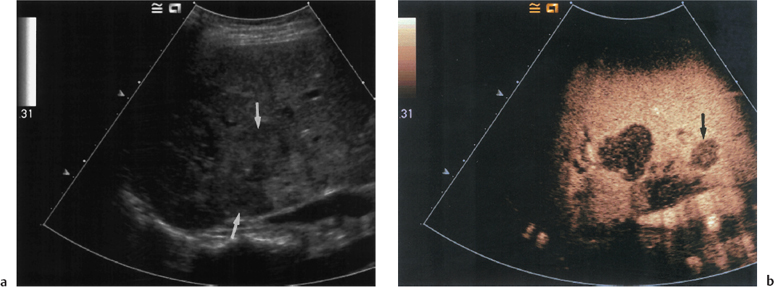
The cirrhotic liver has a coarse, heterogeneous echotexture with increased reflectivity (Fig. 9.1). There is often relative atrophy of segments 4-8, with hypertrophy of the caudate (segment 1) and left lateral lobes (segments 2 and 3) (Fig. 9.2). Although several authors have described the use of various ratios in the diagnosis of cirrhosis,2-6 these are not in common clinical use and are of no relevance in the transplant patient group. Nodularity may be a feature, particularly where histological analysis shows the patient to have a macro-nodular cirrhosis (nodules 3 mm), more commonly seen in chronic viral hepatitis, Wilson’s disease, and alpha1-antitrypsin deficiency.2 Alcohol, biliary obstruction, hemochromatosis, and Budd-Chiari syndrome more commonly give a micronodular cirrhosis, although there is considerable overlap between the two groups (Fig. 9.3).
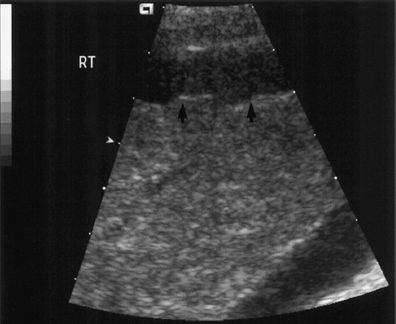
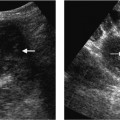
Fig. 9.1 Ascites surrounding a contracted and cirrhotic liver. The liver surface is irregular (arrows) in keeping with macro-nodular change
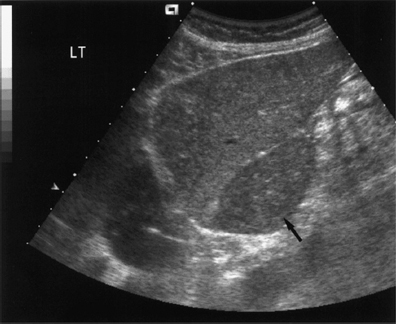
Fig. 9.2 Hypertrophy of the caudate lobe. A hyporeflective enlarged caudate lobe (arrow) in a patient with biopsy-proven liver cirrhosis
Nodules within the liver are classified as regenerative, cirrhotic, dysplastic, or hepatocellular carcinoma (HCC), in accordance with agreed nomenclature.7 It is thought that HCC develops from cirrhotic and subsequently dysplastic nodules, and hence it is of prime importance to try and differentiate these wherever possible. An observational study from Denmark has shown that patients with cirrhosis have an approximately 60-fold risk of developing HCC and a 10-fold risk of cholangiocarcinoma, in addition to variably increased incidence of other alcohol-related and, to a lesser extent, tobacco-related tumors.8
The sonographic appearances of HCC are variable, depending on the size and constitution of the tumor (Fig. 9.4). Small tumors are commonly hypoechoic and well defined, but may be hyperechoic, resembling hemangiomata. As tumors grow, they tend to become more ill defined, with central necrosis giving rise to a more heterogeneous echogenicity. HCC may be solitary, or may be in the form of multifocal nodules. Occasionally, there may be infiltrative disease, giving a diffuse change easily missed on ultrasound.
It is of vital importance that the size and number of tumor nodules are established before the planning of treatment. In patients with a single dominant lesion less than 4 cm in size, transplantation offers potential cure, with one series documenting no recurrence in 14 patients.9 Patients who undergo partial hepatectomy have a poorer prognosis, with more frequent recurrence and 5-year survival rates as low as 25%.10 This is compared with higher disease-free survival at 5 years in transplant recipients (60% versus 14%).11 Recurrence may be due to either undiagnosed “daughter” nodules or de novo malignancy in a susceptible liver. Patients with a tumor larger than 4 cm or multifocal disease are known to do poorly in the postoperative period, with a high incidence of disease recurrence.9 Indeed, transplantation is not recommended in patients with tumor exceeding 5 cm or more than three in number, and is contraindicated in patients with local or systemic extrahepatic spread.12
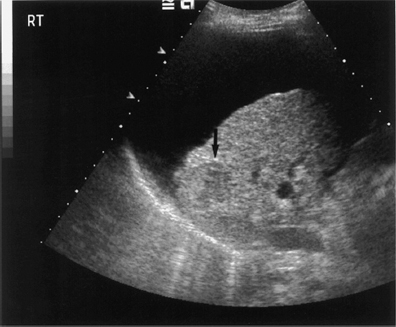
Fig. 9.4 Advanced liver cirrhosis complicated by the development of a hepatocellular carcinoma (HCC) in the right lobe of liver (arrow)
The detection of malignant nodules within the liver with ultrasound has been extensively investigated. Although some authors claim 100% detection of dominant nodules,13 few others have had such success, and a figure of 80% is more common.10,14 One series showed a malignant nodule detection rate of 50% in patients with cirrhotic liver disease.15 It is thought that variation in study populations may account for much of this discrepancy, as not all patients studied had cirrhotic liver disease. By comparison, computed tomography (CT) detects malignancy in 86-100% of cases10,13,14 and hepatic angiography in 90-100%.13,14 The detection of smaller tumors representative of either intrahepatic spread or multifocal primary lesions is much more problematic, with studies showing a detection range from 16%14 to 45%.15 Indeed, all modalities have been shown to be poor in this respect, perhaps the most successful techniques being hepatic angiography and CT aortoportography, although these detect at most 40% of lesions.13
Recent studies with ultrasound microbubble contrast agents have demonstrated a late liver-specific phase three to five minutes after injection. The use of nonlinear methods such as pulse or phase inversion harmonic imaging and stimulated acoustic emission techniques (SAE)16 at this late stage leads to increased conspicuity of focal lesions,17–19 enabling the detection of subcentimeter metastases not detected by conventional ultrasound techniques or CT20,21 (Fig. 9.5). In SAE imaging, HCC and metastases exhibit reduced emission from within the lesions, thought to be due to the absence of Kupffer cells, whereas the presence of high late-phase microbubble contrast uptake is taken as evidence of a benign lesion22 (Fig. 9.6).
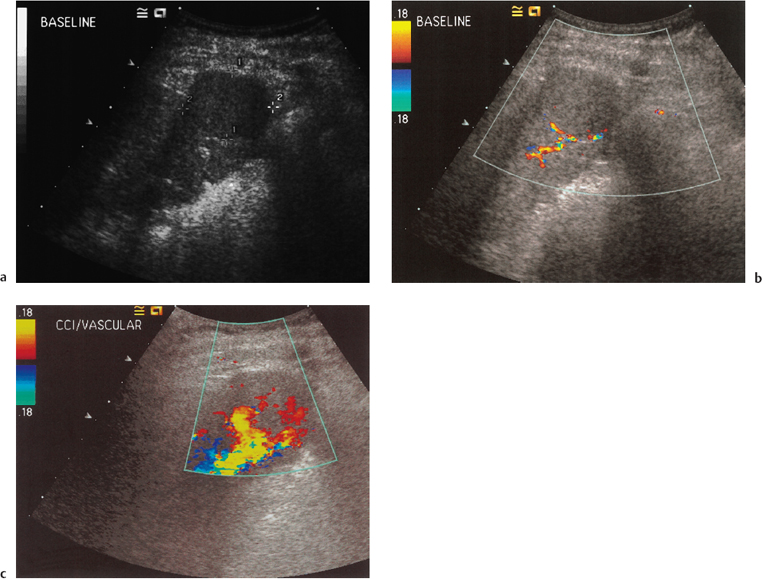
Differentiation of focal nodular lesions in distinct types or categories by ultrasound has been reported as accurate in 98% of patients on the basis of B mode features alone.15 However, other studies have failed to replicate these results. Further attempts at characterizing the lesions by means of tumor vascularity have been reported, but only 76% of lesions known to be HCC demonstrated internal abnormal vascularity in one study.23 Improvements in imaging techniques have resulted in increased flow detection using both power Doppler24 and contrast-enhanced studies,25,26 making assessment of tumor vascularity a potential marker of malignancy23,25,27,28 (Fig. 9.7).
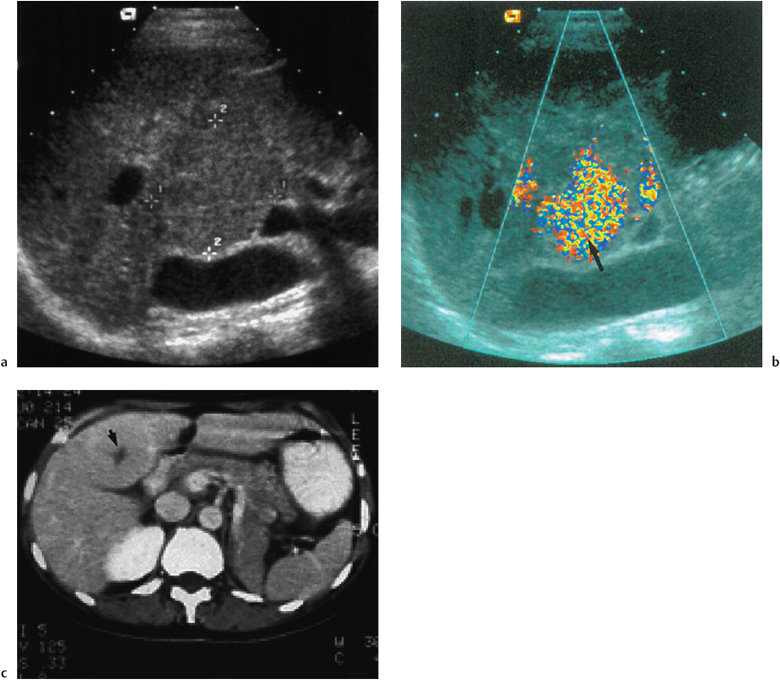
Fig. 9.6 a A low-reflective, well-circumscribed mass (cursors) in the right lobe of the liver in keeping with an area of focal nodular hyperplasia. b Following administration of microbubble contrast (Levovist), imaging in the late phase using stimulated acoustic emission (SAE). Normal functioning tissue shows uptake of the contrast (arrow). c CT (after contrast enhancement) demonstrates uniform enhancement of a focal nodular hyperplaisia except for the “central scar” (arrow)
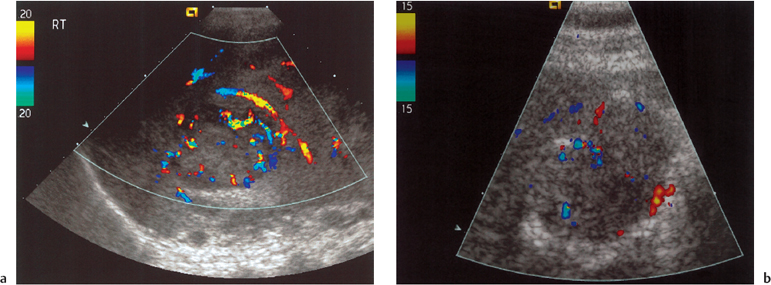
Fig. 9.8 a HCC demonstrating a “basket pattern” of abnormal tumor vascularity on color Doppler imaging. b A metastasis from a primary lung tumor demonstrating a “vessel-within-the-tumor” pattern of vascularity on color Doppler imaging
There are two documented “typical” patterns of vascularity of HCC (Fig. 9.8). The first is a “basket pattern,” where there is encasement of tumor by a feeding artery and portal vein, with both pulsatile and continuous Doppler spectra present. Alternatively, there is the “vessel-within-the-tumor” pattern, characterized by branches of the feeding artery seen within the lesion and displaying a pulsatile Doppler waveform.27 Unfortunately, while these may be indicators of malignant disease where present, there is considerable overlap between HCC and metastases,23 with cavernous hemangiomata also having been reported with abnormal arterial Doppler traces.29
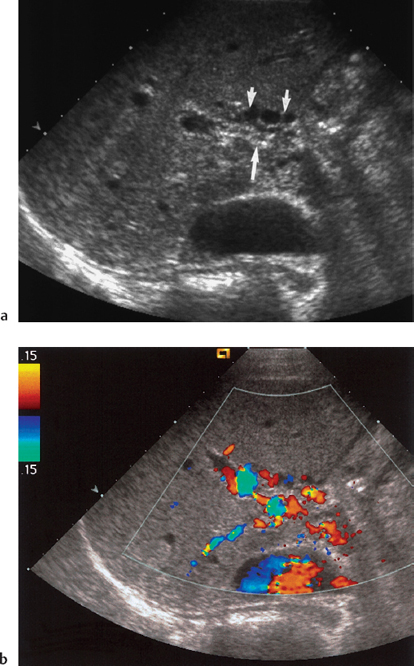
Fig. 9.10 a Attenuated echogenic portal vein (arrow) surrounded by low-reflective areas (short arrow). b Color Doppler ultrasound imaging demonstrates cavernous transformation around a long-standing portal vein occlusion
The presence of a spontaneous arterioportal fistula should suggest the possibility of HCC in patients with cirrhosis, since such fistulae are reported in over 60% of cases. Although shunts may be seen in patients with nontumoral cirrhosis, these are usually subcapsular or peripheral,30 in contrast to the random distribution of HCC-associated shunts.
Cholangiocarcinoma, seen most commonly as a consequence of primary sclerosing cholangitis, may be seen on ultrasound as a hyper- to hypoechoic ill-defined mass, usually in the region of the liver hilum, giving rise to dilated intrahepatic ducts (Fig. 9.9). Further imaging of suspect lesions with endoscopic retrograde cholangiopancreatography allows more accurate assessment of the biliary anatomy and gives the opportunity for diagnostic brushings and palliative stenting of strictures. Proven cholangiocarcinoma is a contraindication to transplantation,12 though patients may be considered for radical resective surgery.
 Summary point:
Summary point:
- The cirrhotic liver has a coarse, heterogeneous echotexture with reduced pulsatility of the hepatic venous waveform
- Ultrasound is approximately 80% sensitive in the detection of HCC. This may be improved by the use of contrast agents
- Differentiation of focal liver nodules by ultrasound is enhanced by the evaluation of the vascular pattern and postcontrast appearances
Imaging the Vasculature
Portal Vein
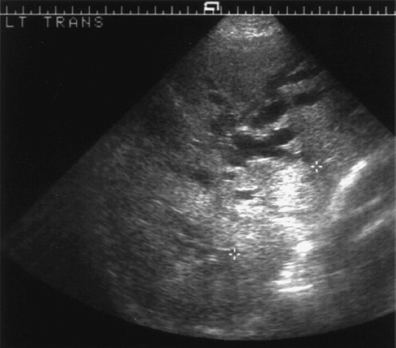
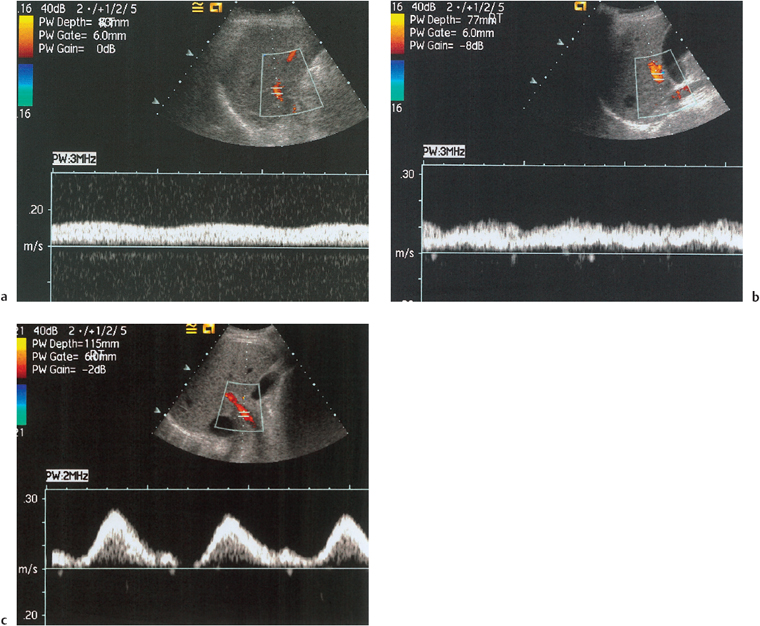
Portal venous thrombosis is a common complication of chronic liver disease, occurring in 5-10% of patients with end-stage cirrhosis31 (Fig. 9.10). Although not an absolute contraindication to transplantation,12 preoperative detection is vital as the full extent of any thrombus needs be demonstrated so that surgery can be planned and techniques modified accordingly.32,33 Portal venous Doppler imaging in normal patients should give a continuous, undulating hepatopetal trace, although there is considerable variation in flow velocity (Fig. 9.11).
In the cirrhotic liver, portal hypertension develops, the increase in pressure accounting for the ensuing complications. Portal velocity and portal venous flow vary inversely with the hepatic venous pressure gradient.34 Recanalization of the umbilical vein in the ligamentum teres may be seen, allowing patency of the portal vein with antegrade flow to be inferred (Fig. 9.12). Reverse flow may also be seen; ultrasound is a reliable indicator of flow direction.35
Portal venous flow characteristics do not predict the presence or otherwise of varices.34 However, among patients in whom variceal bleeding has previously occurred, a 12% or greater reduction in the maximal portal flow velocity four hours after the administration of 40 mg propranolol has been found to be a significant predictor of which patients are less likely to rebleed.36 Portal hypertension may also result in splenomegaly, which may be readily assessed by ultrasound and monitored over time.
B mode imaging of thrombus may show the presence of echogenic material lying within the portal vein, seen as either complete occlusion or a tongue of thrombus with some surrounding flow (Fig. 9.13). Ultrasound is both sensitive and specific in the detection of portal venous thrombosis,37-39 with the small proportion of inaccurate investigations attributed to a diminished flow rate. Demonstration of the portal venous system is enhanced with ultrasound microbubble contrast medium,32,40 ideally given as an infusion,41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree