CHAPTER 51 Atherosclerotic Coronary Artery Disease
Imaging of coronary atherosclerosis has depended on coronary angiography as the gold standard since Sones and Shirey developed the technique at the Cleveland Clinic in the 1960s.1 As pathologic studies have described the stages of atherosclerosis and identified underlying plaque components in areas of thrombosis, the limitations of angiography, which outlines the vessel lumen, have emerged. Currently, we are on the brink of new methods of coronary imaging. Noninvasive transthoracic imaging now rivals angiography for the detection of luminal stenosis, and new catheter-based techniques such as intravascular ultrasound (IVUS) promise to identify the lipid component of plaques. Although the in vivo demonstration of vulnerable plaques, allowing for prevention of plaque progression, is yet elusive, it may be only a matter of time before coronary angiography is replaced by noninvasive techniques as the primary tool for coronary atherosclerotic imaging.
PREVALENCE AND EPIDEMIOLOGY
Coronary atherosclerosis is prevalent in developed countries, with obstructive lesions occurring frequently in individuals older than 50 years. The prevalence is affected by age, gender, genetic predisposition, and acquired risk factors. Although there is a downward trend in the prevalence of heart disease (largely caused by coronary atherosclerosis), it remains the leading cause of death in the United States. The prevalence of coronary heart disease from 1999 to 2004 in the U.S. population, as estimated by the National Center for Health Statistics and National Heart, Lung and Blood Institute, was 22.8% for men and 15.4% for women 60 to 79 years old. Approximately 10% of men 50 to 70 years old who die of noncardiac causes have obstructive coronary lesions at autopsy.2
ETIOLOGY AND PATHOPHYSIOLOGY
Atheromatous Plaque Progression
The classic stages of plaque progression, as defined by the American Heart Association (AHA), involve the formation of the necrotic core. A more recent modification of this staging schema3 outlines development of atheroma. The normal human intima contains a small cushion of smooth muscle cells and matrix, which is present from birth and accentuated at branch points; this lesion has been termed adaptive or diffuse intimal thickening. Later in life, with intimal injury, there is influx of lipids, much of which is oxidized; at this stage (pathologic intimal thickening), there is typically influx of small numbers of foamy macrophages, generally luminal in location to the lipid pools. The progression of the pathologic intimal thickening to fibroatheroma (AHA class II-III) is key in the development of lipid-rich plaques that result in symptoms owing to tendency to rupture and thrombose. The features of fibroatheroma include a core of necrotic material (Fig. 51-1), which is composed of apoptotic cell debris–derived smooth muscle cell membranes, macrophages, and red blood cells. Much current imaging technology is focused on the identification of necrotic material because this is the precursor lesion of the most common substrate for coronary atherothrombosis.
Thin Cap Atheroma and the Vulnerable Plaque
The expansion of the atheroma, or necrotic core of the lipid-rich atherosclerotic plaque, results in thinning of the overlying fibrous cap (see Fig. 51-1). An arbitrary fibrous cap thickness of 65 µm has been designated as the criterion for the “vulnerable” plaque or thin cap fibroatheroma.4 The characteristics of thin cap atheroma that render it prone to rupture are unknown, but likely involve physical factors, such as size of the necrotic core, and biologic factors, such as proteolytic enzyme activity within the thinned cap. The goal of imaging to detect thin cap fibroatheroma and distinguish it from fibroatheroma with thicker caps remains elusive, but may eventually enable the prophylactic treatment of lesions prone to rupture with the aim of preventing atherothrombosis.
Nonatheromatous Plaque Progression
In autopsy studies, approximately 15% of patients who die of coronary artery disease (CAD) lack lipid-rich atheromatous lesions. Nevertheless, these patients develop significant luminal narrowing, and luminal thrombus may occur by mechanisms other than rupture of the thin fibrous cap. The major alternative mechanism of atherothrombosis (Fig. 51-2), termed plaque erosion, is of unclear etiology.5 Erosion may result from endothelial injury that results directly in thrombosis, either via coronary spasm or via interaction with matrix receptors. Because plaque erosion thrombi may be small and nonocclusive, they result in layering of smooth muscle cell–rich plaque that expands without a requisite necrotic core, often with little outward expansion (positive remodeling). Although the goal of imaging remains the detection of lipid-rich plaques, a subset of potentially lethal lesions are not detected by modalities that target lipid.
Life Cycling of the Atherosclerotic Plaque
Because coronary imaging seeks to identify lipid-rich plaques, especially plaques with large necrotic cores, mention of the life cycle of lipid-rich lesions and the effect of successive ruptures on plaque composition is relevant. One outcome of plaque rupture, after slowly enlarging core and thinning of the fibrous cap, is occlusive thrombus that organizes as a total occlusion and results in acute coronary syndrome. Much more common after acute rupture is a subocclusive thrombus with healing. The outcome of subocclusive plaque rupture is often plaque enlargement and outward expansion (remodeling) of the arterial wall. The thickening of the fibrous cap that occurs after healed rupture is roughly represented by the AHA class V plaque. Autopsy studies have shown multiple old rupture sites at sites of acute rupture, resulting in compartmentalization of the plaque into areas of lipid core and fibrous healing.6 The site of future rupture may not have a single large lipid compartment, but a heterogeneous makeup that includes fibrous tissue. The consequences for imaging are unclear at this time.
Calcification and Coronary Atherosclerosis: Implications for Imaging
Calcification is a reliable marker for the presence of atherosclerotic plaque because there are no other processes in the coronary arteries that result in calcium deposition. Calcium scoring is a reliable marker for atherosclerotic plaque burden, although in younger patients severe obstructive lesions can occur in the absence of any calcification (see Chapter 32). Calcification is not a marker of plaque instability, however. Solid calcification can be the result of diffuse deposition in fibrous tissue in stable plaques, whereas more stippled calcification, reflective of necrotic core calcification in more heterogeneous plaques, may be more suggestive of an unstable plaque.7
MANIFESTATIONS OF DISEASE
Clinical Presentation
The major clinical presentations of coronary atherosclerosis are stable angina, acute coronary syndromes (ST segment elevation MI and non–ST segment elevation MI), congestive heart failure, and sudden death. Acute coronary syndromes are discussed Chapter 52. Coronary lesions in sudden death are quite heterogeneous because the mechanisms of ventricular arrhythmias vary. In stable angina, there is generally stable plaque with significant obstruction, whereas unstable syndromes are characterized by intraluminal thrombus. In unstable angina or non–ST segment elevation MI, typically nonocclusive thrombi are present, whereas ST segment elevation MI is associated with larger, often occlusive thrombi. Coronary artery lesions in ischemic cardiomyopathy are typically diffuse and multiple, and there is significant myocardial damage generally with transmural healed MI.
Imaging Indications and Algorithm
Currently, angiography is the gold standard for imaging atherosclerotic coronary disease, and other imaging techniques are investigational. CT scanning for coronary calcium is used as identification for coronary risk (see Chapter 32), and IVUS is indicated for the evaluation of graft vascular disease in heart transplant recipients.
The indication for coronary angiography varies by clinical setting. The AHA has set forth algorithms for coronary angiography that are stratified by specific patient groups.8 Patients in whom coronary arteries should be evaluated by angiography include patients with symptoms of stable angina, nonspecific chest pain, unstable angina, recurrence of ischemia after revascularization, and acute MI, and patients undergoing perioperative risk assessment for noncardiac surgery. Patients with valvular heart disease in whom surgery is contemplated, patients undergoing cardiac surgery for other indications including congenital heart disease, and patients with unexplained congestive heart failure may also be candidates for coronary angiography. There are no currently accepted indications for coronary artery imaging other than angiography, although noninvasive modalities, especially multislice CT, are on the brink of being established as a viable option for the identification of coronary artery stenoses (see later).
Imaging Technique and Findings
Angiography
Catheter-based invasive coronary angiography is the gold standard for diagnosing significant (≥50% diameter stenosis) CAD. Despite various, less invasive tests to detect CAD, including stress imaging studies, 35% of patients referred for catheter-based angiography have no significant stenoses, stressing the need for better noninvasive tests. The rate of false-negative results of coronary angiography is not well appreciated, however; one of the few anatomic correlations showed that angiography significantly underestimates lesions in 44% of cases.9 Degree of stenosis as measured by coronary CT angiography shows little correlation between functional severity as measured by fractional flow reserve.10 These findings suggest that angiography is not ideal for identifying coronary stenosis, and that the mere detection of luminal narrowing by even more sophisticated methods may not be as important as functional studies or imaging techniques that show lesion characteristics.
The need to identify functional characteristics of coronary lesions is borne out by follow-up studies. Although the incidence of progression of thin cap atheroma is unknown, at least 6% of patients undergoing percutaneous coronary intervention develop progression of disease requiring intervention for a different target lesion within 1 year.11 The major predictors of plaque progression are multivessel disease, prior percutaneous coronary intervention, and age younger than 65 years. It has also been shown that 17% of acute MI patients with multiple complex angiographic lesions undergo percutaneous coronary intervention of nonculprit lesions within 1 year.12 These data underscore the need for identifying plaques in high-risk patients who would benefit from prophylactic intervention.
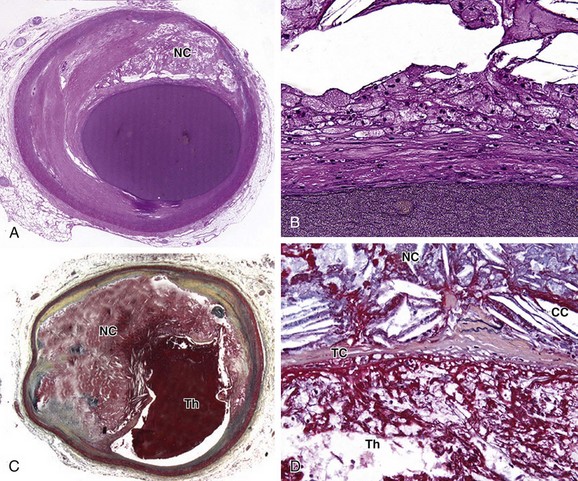
 FIGURE 51-1
FIGURE 51-1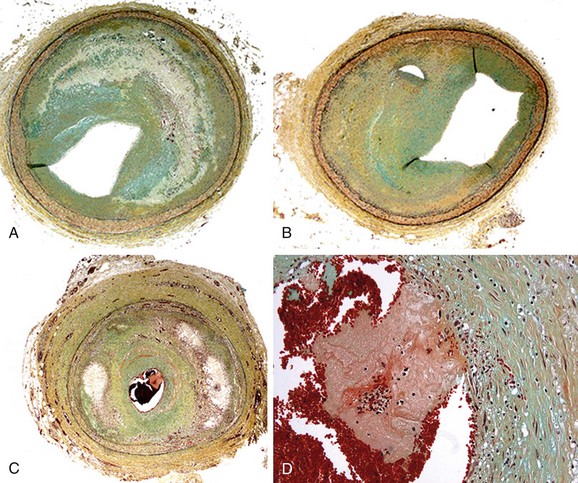
 FIGURE 51-2
FIGURE 51-2


