and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
4.1 Introduction: Cancer Treatment with Radioisotopes
The potential use of Auger emitters for cancer therapy is important, since these radioisotopes decay with emission of Auger electrons which deposit very high energy over very short distances. In this manner, the use of selected Auger emitters conceptually represents an important alternative to the use of alpha-emitting and beta-emitting radioisotopes for cancer therapy. Interest in the use of Auger-emitting radioisotopes for cancer therapy is continually increasing partly because of the development of complementary targeting technologies for cell-specific delivery. However, an important which must also be addressed is the availability of the required Auger emitters.
The common treatment of cancerous tumors that cannot be surgically removed usually involves chemotherapy and external beam irradiation. When access is possible, sealed radioactive sources administered by a radiation oncologist (“brachytherapy”) is another major treatment option. An additional approach employs the use of radiopharmaceutical agents labeled with unsealed radioactive sources (“radiopharmaceuticals”) as discussed earlier in Chap. 1. In this technology for cancer therapy, for instance, therapeutic radioisotopes are attached to targeting molecules designed for site-specific tumor cell binding. These agents are injected intravenously and thus act as carriers for targeting of the therapeutic radioisotopes to cancer cells. The use of therapeutic radioisotopes in this manner can minimize many of the side effects encountered with many traditional treatment methods. This unique opportunity for site-specific targeting of cancer cells for therapy represents a major clinical weapon for the treatment of cancer. Specifically targeted antibodies for treatment of non-Hodgkin lymphoma cancer radiolabeled with the yttrium-90 (Zevalin®) and iodine-131 (Bexxar®) beta-emitting radioisotopes had been approved as the first agents for routine clinical use by the US Food and Drug Administration (FDA).
4.2 Particle Emission
The identification of the type of radiation or radioisotope best suited for treatment of a neoplasia is dependent upon a number of factors, which include the type of cancer, its staging, the size of the treatment area, the depth of radiation penetration required, and the location of the cellular sites for binding of the radioactive targeting agents. Traditionally, the use of high-energy beta-particle-emitting radioisotopes such as 90Y, for instance, has permitted deep penetration (millimeters) into solid tumors and utilization of the effects of “cross fire” for the irradiation of target cells which may be even millimeters distant from the binding site (Table 4.1). The use of high penetration permits energy deposition to kill cells to which the targeting molecule may not bind (i.e., “cross fire”), but normal cells can also receive damaging levels of radiation. This can often be important because of the cellular heterogeneity of solid tumors, where normal cells and cancer cells are intermingled. However, at least theoretically, the possible therapeutic use of Auger emitters has at least theoretically many advantages in comparison to beta- and alpha-emitting radioisotopes (Behr et al. 2000; Humm et al. 1994; Sastry 1992).
Table 4.1
Comparison of relative maximal soft tissue penetration and energy deposition of high-energy beta particles, alpha particles, and Auger electrons used in cancer therapy
Type of particle emission | Example of radioisotope | Energy of emission | Approximate soft tissue penetration |
|---|---|---|---|
Beta particle (β) | Yttrium-90 | 2 MeV | 10 mm |
Alpha particle (α) | Bismuth-213 | 8 MeV | 4–5 cell diameters |
Auger electron (AE) | Indium-111 | <eV | Confined within single cell |
4.3 The Auger Process
When radionuclides decay by electron capture (EC), e.g., 55Fe, or by internal conversion (IC), e.g., 195m Pt, a vacancy is created in the inner atomic shell of the residual atom. Subsequently, the excited atom undergoes a series of radiative and non-radiative transitions until the ground state of the atom is reached. Radiative transitions predominate for K-shell vacancies and result in the emission of a characteristic X-ray (see Fig. 4.1). Although there are a variety of non-radiative transitions, the most common are the Auger, Coster–Kronig (CK), and super Coster–Kronig (super CK) transitions as shown in Fig. 4.1. Non-radiative transitions are highly probable when the vacancy lies beyond the L-shell. In an Auger transition, an initial electron vacancy in a lower shell is filled by an electron from a higher shell and another electron is ejected from a higher shell. While CK transitions are similar, the electron that fills the vacancy originates from the same shell and the ejected electron is from a higher shell. In super CK transitions, the initial vacancy and the two electrons are all in the same shell. Radiative transitions move the electron vacancy to a higher shell with no change in the number of vacancies; with nonradiative transitions, the number of vacancies increases by one. As the innermost vacancy percolates towards the valence shell, a cascade phenomenon develops with corresponding vacancy multiplication and emission of numerous low-energy electrons, collectively referred to as Auger electrons. Most of the electrons have very low energies (less than a few hundred eV), intermediate values of linear energy transfer (10–25 keV μm−1), and extremely short ranges (several nm) in biological matter. Since this electron cascade process is completed with ~10–15 s, the region in the immediate vicinity of the atom is showered with Auger electrons and, as a consequence, very high local energy densities are realized. It is this intense and localized energy deposition which we wish to exploit as an approach for cancer therapy.
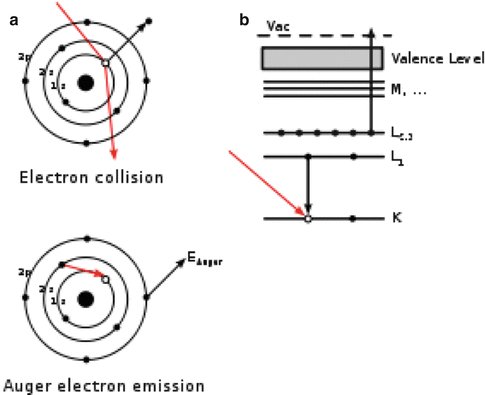

Fig. 4.1
Representation of the Auger cascade process
4.4 Cell Killing with Auger Electron Emitters
In contrast to the use of high-energy beta particles, the high linear energy transfer (LET) alpha particles and Auger electrons have significantly lower soft tissue penetration (Table 4.1) and thus deposit all their energy in much shorter distances (microns or less). For this reason, the necessity of specific cancer cell targeting is much more important. If most of the tumor cells are accessible, carrier molecules labeled with alpha- and Auger electron-emitting radioisotopes can thus be targeted for transport within the target tumor cells or ideally to the target cell nucleus. In this manner, the limited range of these high LET emissions can be effectively used for cell killing. In this context, the optimal therapeutic approach for cancer therapy using unsealed radioactive sources which target tumor cells is the use of low LET beta-emitting radioisotopes for the treatment of the large (primary) tumor masses combined with the use of high LET (alpha or Auger) radioisotopes for therapy of small, cellular cluster metastatic sites. As an example, only recently, the internalization of peptides which specifically bind to receptors on cancer cells has been established, thus providing an effective targeting tool for delivery of these high LET radioisotopes. A commercially available octreotide analog, “Octreoscan” (111In-Pentetreotide; Phe-D-octreotide), is used for somatostatin receptor scintigraph for the detection and localization of primary tumors and metastatic spread which are often missed by conventional imaging modalities (Krenning et al. 1996). Such octreotide analogs are known to internalized within the cell after binding to receptors expressed on the cell surface, and moreover, the therapeutic efficacy of In-111-Pentreotide has been demonstrated in high-dose studies, demonstrating the therapeutic effectiveness of intracellular Auger electron emission in both animal tumors models (de Jong et al. 1998) and in human tumors in vivo (Krenning et al. 1996), since 111In decays with the emission of 34 KeV Auger electrons (Average Energy). It is important to note in this context that 111In-labeled octreotate does not localize in the cell nucleus but remains in the cytoplasmic compartment after internalization. This observation would indicate that cytoplasmic targeting of radiopharmaceuticals labeled with Auger emitters can be therapeutically effective and that nuclear targeting is always not the key. However, 111In may be impractical for routine clinical therapy because of its limited availability and very high production costs (Fig. 4.2).
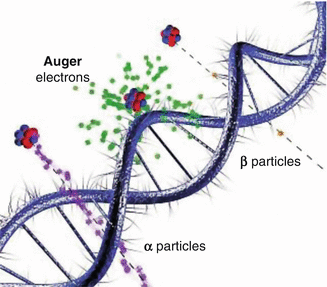

Fig. 4.2
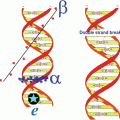
Illustration of the relative interaction of Auger electrons with the double helix of the DNA molecule, in comparison with alpha (α) and beta (β) particles (Taken from Web site of, Department of Nuclear Physics Research School of Physics & Engineering ANU College of Physical & Mathematical Sciences)
This observation of the successful intracellular targeting of radiolabeled peptide is an important observation, and the use of Auger-emitting radioisotopes could thus be an attractive future widespread approach for tumor therapy. The ability to target radiolabeled peptides or other molecules to the cell interior suggests the important use of Auger electron-emitting radioisotopes for therapy. Just as clinical applications of alpha-emitting radioisotopes (Chap. 3) such as 225Ac, 213Bi, and 223Ra have been demonstrated for the specific application for localized sterilization of small clusters of cancer cells, the next stage of development would be the expected use of Auger electron emitters for targeting of single cells to augment the use of lower LET radioisotopes and other strategies for cancer therapy.
An understanding of the transport of low-energy electrons in soft tissue is also an important issue for dose planning, safety concerns, and relating targeting with therapy response. There is broad interest from dosimetric, therapeutic, and clinical perspectives regarding the use of Auger-emitting radioisotopes coupled to specific cellular/nuclear targeting vectors for cancer therapy. The highest radiobiological effectiveness (RBE) results when Auger emitters are incorporated into the highly radiosensitive cell nucleus (Daghighian et al. 1996). Results of studies with the Auger electron-emitting radioisotope 125I clearly demonstrate the severe radiotoxicity when a 125I-labeled nucleic acid precursor (e.g., 5-125I-iododeoxyuridine, a thymidine analog) is incorporated into the DNA of proliferating cells (Kassis et al. 1987). These studies have demonstrated the important potential for the use of Auger electron emitters for the treatment of cancer. However, while the use of the 125I continues to be of importance for basic studies, this radioisotope is impractical for clinical use. There are thus a limited number of Auger emitters which can be practically produced and have the appropriate decay and chemical properties. As summarized in Table 4.2, only a few Auger emitters are probably of practical importance for clinical applications, and examples can be either reactor or accelerator produced. Two key reactor-produced Auger emitters of current interest are platinum-195m (195mPt) and rhodium-103m (103mRh). The number of Auger electrons emitted per each radioactive disintegration (Ne/Nt) is very high for 195mPt. The high auger yield from decay of 103mRh coupled with its carrier-free availability from a generator system and its well-known chelate chemistry—required for attachment to cancer cell targeting molecules—make this a key candidate for further studies. As the case for all targeted radiopharmaceuticals, a key challenge is the effective high-specific-activity radiolabeling required for targeting molecules to evaluate the potential effectiveness of such agents.
Table 4.2
Key examples of radioisotopes emitting secondary electrons that have potential applications for intracellular auger therapy
Radionuclide production | Half-life | Emission | keV | <e> (keV) | Ne/Nt | Eγ (Iγ) keV (%) | Daughter radioisotope |
|---|---|---|---|---|---|---|---|
103Ru R | 39.4 days | β | 763 | 41.7 | NA | 497 (86.4) | 103mRh (56.1 m) |
103Pd R, A | 17.0 days | EC | 546 | 42.5 | NA | 357 (0.02) | 103mRh (56.1 m) |
103mRh R | 56.1 m | IT | 43 | 37.5 | 5.71 | 40 (0.07) | 103Rh (stable) |
111In A | 2.8 days | EC | 850 | 33.9 | 7.2 | 171.3 (90.2) 254.4 (94.0) | 111Cd (stable) |
123I A | 13.2 h | EC | 1200 | 27.6 | 13.7 | 159 (83.3) | 123Te (1.3 × 1013 years) |
125I R | 60.3 days | EC | 178 | 17.9 | 22.9 | 35 (6.7) | 125Te (stable) |
161Ho A | 2.48 h | EC | 25.7 | … | 161Dy (stable) | ||
193mPt R | 4.33 days | IT | 150 | 130 | 21.8 | 135 (0.11) | 193Pt (50 years) |
195mPt R | 4.02 | IT | 260 | 175 | 35.9 | 99 (11.4) | 195Pt(stable) |
193mHg A | 1.73 days | IT (54 %) EC (46 %) | 176 1520 | 139.6 | NA | 262 (32.4) 560 (7.5) | 195Hg (9.5 h) |
195Hg A | 9.5 h | EC | 1520 | 61.3 | NA | 780 (7.0) | 195Ag (185 days) |
197mHg A | 23.8 h | IT (93 %) EC (7 %) | 299 600 | 214 | NA | 140 (34)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|