Fig. 17.1
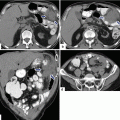
Autoimmune pancreatitis macroscopic appearance. Photographs of two bivalved specimens from distal pancreatectomies (a, b) show a white, firm, ill-defined mass of dense fibrotic tissue replacing the normal pancreatic parenchyma (arrows)
Gray to yellowish-white induration of the affected tissue with loss of its normal lobular architecture.
Involved portions of the pancreas may be enlarged.
Diffuse involvement of the pancreas may also be found.
Gross appearance mimics pancreatic ductal adenocarcinoma because this inflammatory process, like pancreatic carcinoma, commonly presents on the head of the pancreas.
Can be associated with obstruction of the main pancreatic and distal bile ducts.
Unlike other types of chronic pancreatitis, there are no associated pseudocysts, fat necrosis, or inspissated secretions.
Pancreatic calcifications are usually absent, although they may occur late in the course of the disease.
17.3.2 Microscopic Appearance
Autoimmune pancreatitis is divided into two clinicopathologic subtypes: type 1 AIP and type 2 AIP.
17.3.2.1 Type 1 AIP (Fig. 17.2)
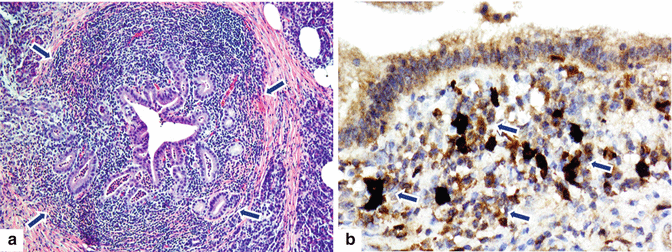
Fig. 17.2
(a, b) Autoimmune pancreatitis type 1, microscopic appearance. Histological section (H&E, 10×) (a) shows a prominent lymphoplasmacytic infiltrate surrounding the ducts and the acini in a nodular pattern with reactive stromal fibrosis (arrows ). Immunohistochemistry for IgG4 (b) highlights (arrows) the increased number (>10/HPF) of positive plasma cells (arrows) responsible for this type of autoimmune pancreatitis
Also called lymphoplasmacytic sclerosing pancreatitis (LPSP).
Characterized by a diffuse lymphoplasmacytic infiltrate with increased IgG4-positive plasma cells and associated storiform-type fibrosis surrounding medium and large interlobular ducts.
Obliterative venulitis with lymphocytes and plasma cells.
Abundant (>10 IgG4 positive plasm cells per high-power field)
Smaller ducts are involved in advanced cases.
May contain some macrophages and, occasionally, few neutrophils and eosinophils.
Immunocytochemical phenotype: most lymphocytes are CD8- and CD4-positive T lymphocytes, with scattered CD20 + B lymphocytes.
Infiltrate completely encompasses the ducts and may narrow their lumen by infolding of the epithelium (star-like structure).
In later stages, the ductal wall is thickened by periductal fibrosis.
17.3.2.2 Type 2 AIP (Fig. 17.3)
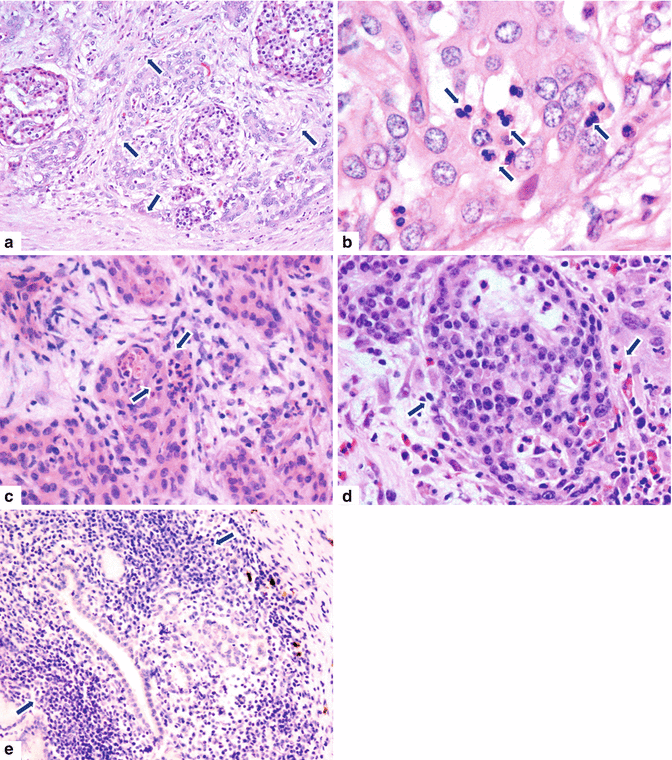
Fig. 17.3
Autoimmune pancreatitis type 2, microscopic appearance. Histological sections show: (a) (H&E, 10×) an acute inflammatory infiltrate involving the acinar and islet cells with disruption of the normal architecture, producing stromal fibrosis (arrows); (b) (H&E, 100×) shows the characteristic granulocyte epithelial lesion (GEL) in the acinar cells, which show reactive nuclei and architectural disarry (arrows); (c, d) (H&E, 40×) shows the GEL and reactive stromal fibrosis (arrows); and (e) (H&E, 10×) shows a dense periductal lymphoplasmacytic infiltrate (arrows)
Also called idiopathic duct-centric pancreatitis (IDCP) or AIP with granulocytic epithelial lesions.
Shows periductal lymphoplasmacytic infiltrate and storiform fibrosis.
Characterized by the presence of prominent neutrophilic and lymphoplasmacytic infiltrate in the medium- and small-sized ducts, as well as in acini forming microabscesses, ductal ulcerations, and granulocytic epithelial lesions (GEL). If present, only occasional (<10/HPF) IgG4-positive plasma cells are seen.
Practical Pearls
Extension and severity of chronic changes vary from case to case and even from one area to another within the same pancreas.
In addition to ductal and sclerotic changes, there are vascular changes.
Vasculitis affecting the small veins is the most frequent.
If the inflammatory process involves the head of the gland (80 %), it usually involves the distal common bile duct (diffuse lymphoplasmacytic infiltration combined with storiform-type fibrosis).
In some cases, the inflammation also extends to the hepatic ducts of the liver hilus and gallbladder wall.
17.4 Clinical Presentation
Typical presentation: abdominal pain, painless jaundice with fatigue and weight loss.
AIP occasionally presents with classic signs and symptoms of acute pancreatitis.
Other clinical manifestations include diabetes mellitus or steatorrhea.
17.4.1 Type 1 AIP
Usually seen in older men (60–70 years old)
Male to female ratio of 2:1
Most patients have elevated serum IgG4 concentrations
Commonly associated with extrapancreatic involvement such as:
Sclerosing cholangitis
Sialadenitis (Sjogren’s syndrome)
Lacrimal gland and salivary gland swelling (Mikulicz syndrome)
Abdominal, retroperitoneal, hilar, or mediastinal lymphadenopathy
Autoimmune thyroiditis
Interstitial nephritis
Retroperitoneal fibrosis
Vascular disease
Pulmonary nodules, mass-like lesions
17.4.2 Type 2 AIP
Affects younger individuals
Not gender predominant
Commonly associated with inflammatory bowel disease
Serum IgG4 levels usually normal
Practical Pearls
The most common presentation of AIP mimics that of pancreatic cancer; thus, a correct diagnosis of AIP can avoid major surgery.
The diagnosis is challenging, because its incidence is far lower than that of the disease it mimics and there is no single diagnostic clinical feature or test that can identify the full spectrum of AIP.
A characteristic feature, which can serve as a clue in the diagnosis of AIP, is the involvement of the other organs.
17.5 Laboratory Evaluation
Elevated Serum Antibodies
A serum concentration of IgG4 that is ≥2 times the upper limit of normal is highly suggestive of AIP type 1, but does not rule out pancreatic cancer.
A serum concentration of IgG4 usually is normal in type 2 AIP.
Other serum antibodies that may be elevated:
Anti-nuclear
Anti-lactoferritin
Anti-smooth muscle
Anti-carbonic anhydrase II
If associated with acute pancreatitis:
Elevated serum lipase and amylase levels
If associated with biliary obstruction:
High bilirubin levels
High alkaline phosphatase levels
17.6 Imaging
Preferred imaging modalities: CT or MRThe most commonly described imaging findings of AIP can be divided into typical or atypical and can be identified using different imaging modalities.
Typical Findings
Diffuse enlargement of the pancreas is seen in 40–60 % of the patients.
Effacement of the lobular contour of the pancreas.
Capsule-like rim with low attenuation surrounding the enlarged pancreas.
Atypical Findings
Focal or multifocal enlargement of the pancreas
No deforming mass or hypoattenuated-like, geographical areas in the pancreatic parenchyma
17.6.1 Ultrasound (US)
Conventional
Endoscopic (EUS)
Findings
Diffuse type (Figs. 17.4–17.6)
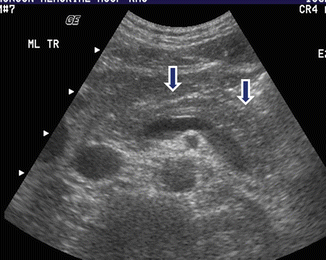
Fig. 17.4
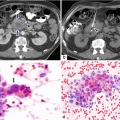
Autoimmune pancreatitis on US. A 68-year-old male with abdominal pain, weight loss, and elevated serum IgG4. Transverse image shows a mild atrophic pancreas with smooth margins (arrows)
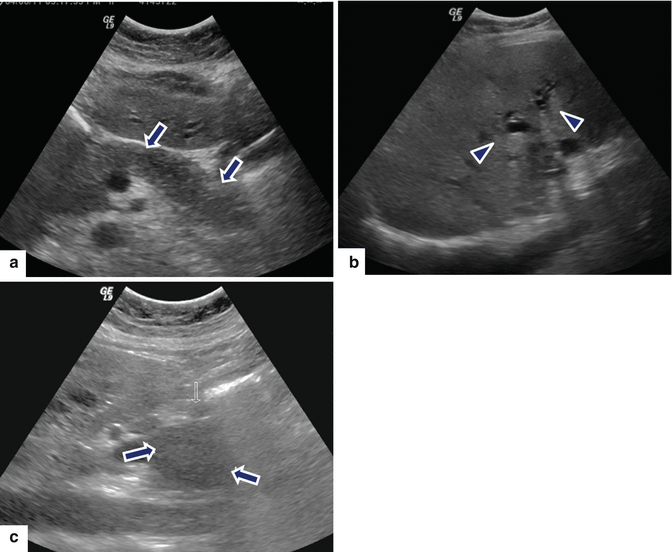
Fig. 17.5
Diffuse autoimmune pancreatitis associated with obstruction of the biliary system. A 32-year-old male with epigastric pain, jaundice, and weight loss. Transverse (a, b) and sagittal (c) images demonstrate a hypoechoic pancreas (arrow) with a prominent head (b) (arrows) associated with obstruction of the common bile duct and dilatation of the intrahepatic biliary system (arrowheads)
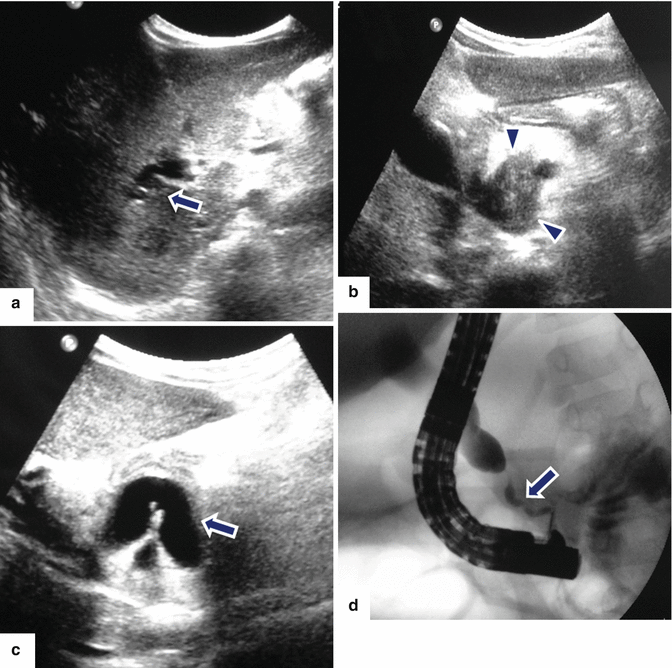
Fig. 17.6
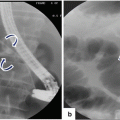
Autoimmune pancreatitis on US and ERCP. A 2 year old female patient who presented with jaundice, and elevated bilirubin and alkaline phosphatase. Transverse (a–b) and sagittal (c) images demonstrate a large hypoechoic mass in the head of the pancreas (b) (arrowheads) associated with obstruction of the intra and extrahepatic biliary system (a, c) (arrows). ERCP (d) image reveals a long irregular stricture of the most distal common duct (arrow). Note the dilatation of the common bile duct proximal to the stenosis (arrow). Due to a presumptive malignant pancreatic mass, the patient underwent a pancreaticoduodenectomy. Final pathology diagnosis: Autoimmune Pancreatitis
Diffuse enlargement or atrophy of the pancreatic parenchyma with hypoechoic appearance and smooth margins
Can be associated with obstruction of the pancreatic or common bile duct
Focal type (Figs. 17.7–17.9)
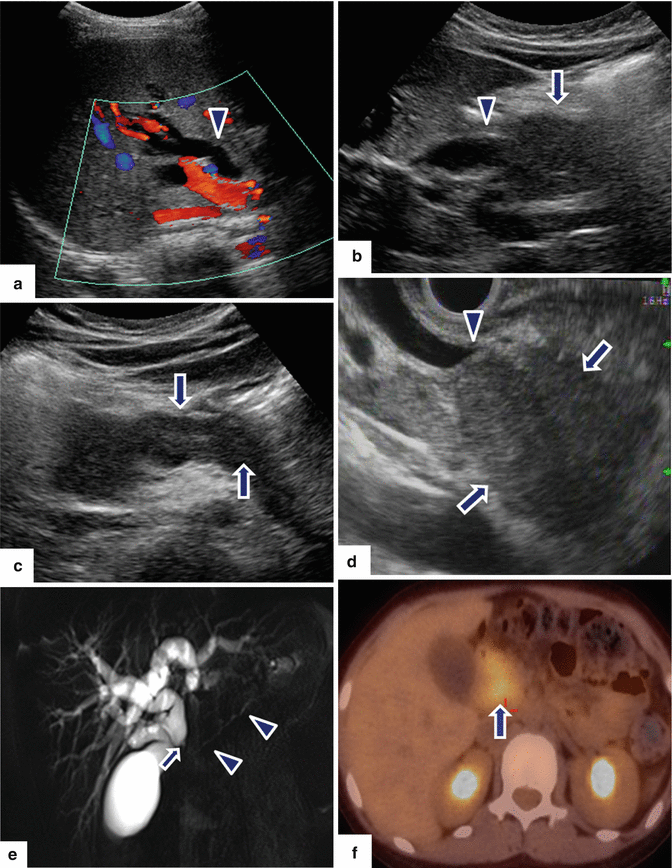
Fig. 17.7
Autoimmune pancreatitis on US/MRCP/PET/CT 8 year old female with history of diarrhea, steatorrhea and weight loss. Tranverse scans (a–c) show a diffuse featureless, enlarged hypoechoic pancreas (c) (arrows). Note the dilatation of the extrahepatic biliary system up to the level of the head of the pancreas (a, b) (arrowheads). The patient underwent a fine needle aspiration under endoscopic ultrasound (EUS). EUS (d) shows uniform hypoechoic mass in the head of the pancreas (arrows) obstructing the common bile duct (arrowhead). MRCP thick slab (e) shows dilatation of the intra and extrahepatic biliary system with tapering of the bile duct at level of the pancreatic head (arrow) and a beaded appearance of the main pancreatic duct (arrowheads). PET/CT axial (f) image shows hypermetabolic uptake in the pancreatic head (arrow). Final pathology from needle aspiration: Autoimmune pancreatitis
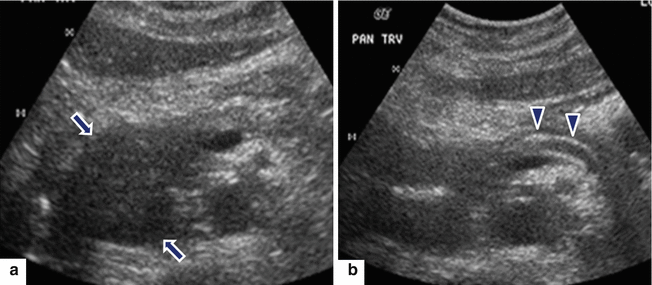
Fig. 17.8
Focal pancreatitis on IOUS. A 69-year-old male with mild epigastric pain and weight loss. Transverse scans (a, b) reveal a well-defined mass in the head of the pancreas (arrows) associated with dilatation of the main pancreatic duct and atrophy of the body of the pancreas (arrowheads)
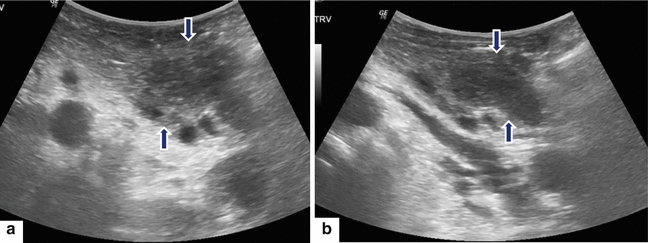
Fig. 17.9
Focal autoimmune pancreatitis on IOUS. A 31-year-old patient with repetitive attacks of acute pancreatitis. IOUS transverse (a, b) images demonstrate an ill-defined hypoechoic mass involving the tail of the pancreas (arrows). The patient underwent a distal pancreatectomy and splenectomy. Final pathology: focal autoimmune pancreatitis
Focal hypoechoic, well- or ill defined enlargement of the pancreas
Linear or reticular (tortoise-shell pattern) hyperechoic inclusions (EUS)
The main pancreatic duct may be found to penetrate through the mass (pancreatic duct-penetrating sign)
17.6.2 Computed Tomography (CT)
Findings
Diffuse type (Figs. 17.10–17.17)
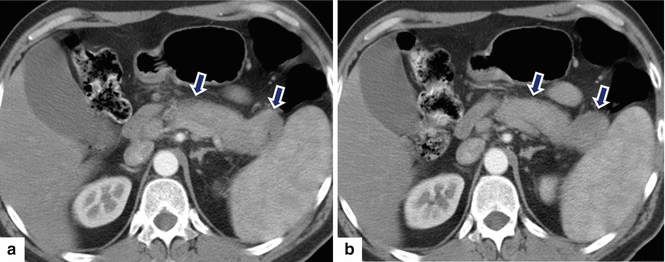
Fig. 17.10
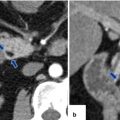
Autoimmune pancreatitis on CT. A 54-year-old male with abdominal pain. CECT axial (a, b) images show a heterogeneous prominent, featureless pancreas with very mild peripancreatic hypodense halo (arrows)
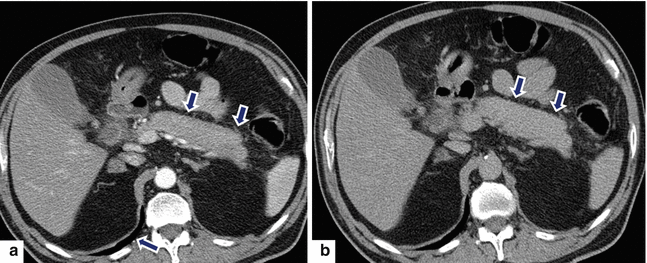
Fig. 17.11
Autoimmune pancreatitis on CT. A 79-year-old female with malaise and weight loss. CECT arterial (a) and delayed phase (b) axial images demonstrate a featureless pancreas with mild peripancreatic inflammatory changes (arrows)
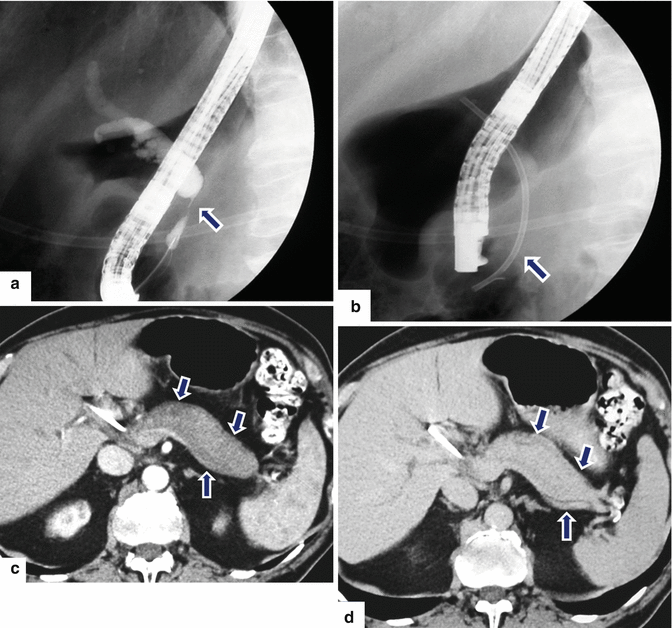
Fig. 17.12
Autoimmune pancreatitis on CT. An 84-year-old male with jaundice and abdominal discomfort. Patient underwent an ERCP (a) that demonstrates dilatation of the intra- and extrahepatic biliary system with abrupt cutoff of the common bile duct in the head of the pancreas. Note the presence of a smooth stenosis of the common bile duct at this level (arrow). The biliary stenosis was bypassed with a stent (b). CECT (c) arterial phase images reveal an enlarged, heterogeneous, featureless pancreas with sausage-like appearance (arrows). Delayed phase image (d) reveals homogeneous enhancement of the pancreatic parenchyma and the presence of a peripancreatic thick low-density rim (arrows). Note the presence of a biliary stent in the decompressed common bile duct
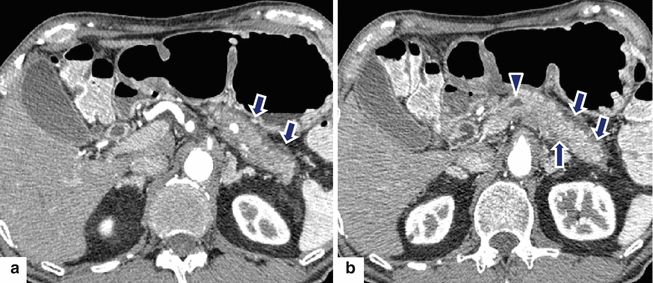
Fig. 17.13
Autoimmune pancreatitis on CT. A 77-year-old male with history of abdominal pain and 10 lb weight loss. CECT axial (a, b) images show atrophy of the pancreas, mild segmental dilatation of the pancreatic duct (arrowhead), and a thin low-density capsular rim surrounding the pancreas (arrows)
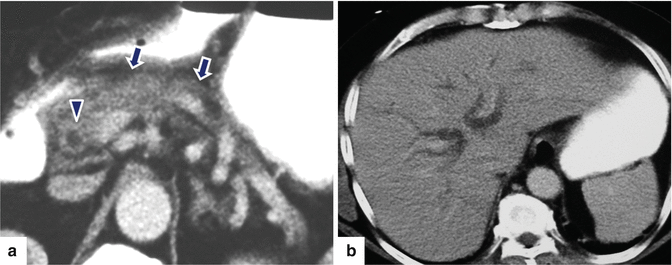
Fig. 17.14
Autoimmune pancreatitis on CT. A 54-year-old male with painless jaundice. CECT axial (a, b) images demonstrate an atrophic featureless pancreas (arrows) and dilatation of the intra- and extrahepatic biliary system up to the level of the head of the pancreas. Note the periductal circumferential wall thickening of the common bile duct (a) (arrowhead)
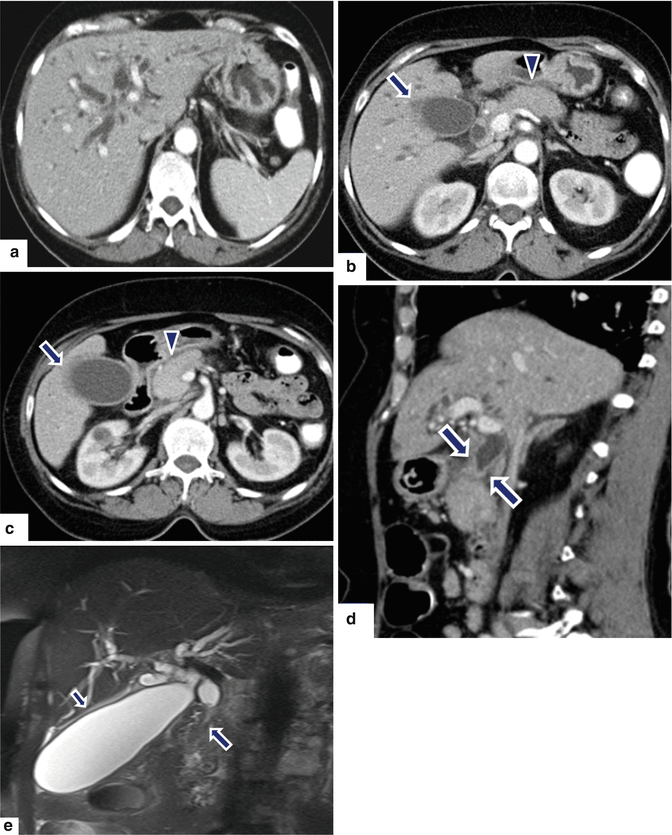
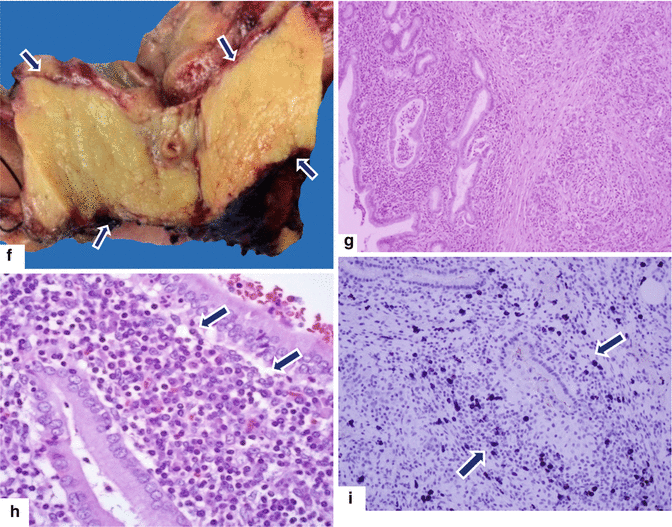
Fig. 17.15
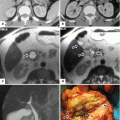
Focal autoimmune pancreatitis on CT. A 57-year-old female with history of breast carcinoma with acute onset of jaundice. CECT axial (a–c) and sagittal (d) images demonstrate a featureless pancreas (b, c) (arrowheads) and dilatation of the biliary system secondary to the obstruction of the common bile duct in the head of the pancreas (a, d). Note the thick enhancing wall of the common duct and gallbladder (c, d) (arrows). Single-shot fast spin echo T2-weighted coronal image (e) demonstrates tapering of the distal common bile duct at the level of the head of the pancreas with secondary obstruction of the biliary system (arrow). This study confirms the mild thickening of the gallbladder (short arrow). The patient was taken to the OR to perform a biliary bypass for a presumptive diagnosis of metastatic breast carcinoma. At surgery a firm mass was palpated in the head of the pancreas. The patient underwent a Whipple procedure. Photograph of the bivalved specimen (f) demonstrates a hard, yellow mass in the head of the pancreas (arrows). Histological sections (g, h) (H&E, 4 & 40×) demonstrate prominent lymphoplasmacytic infiltrate surrounding the pancreatic ducts (h) (arrows) and disrupting the acini. Immunohistochemistry for IgG4 (i) highlights an increased number of positive plasma cells above the cut-off (>10/HPF) (arrows) for the diagnosis of AIP type 1. Immunoperoxidase, 10×
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree