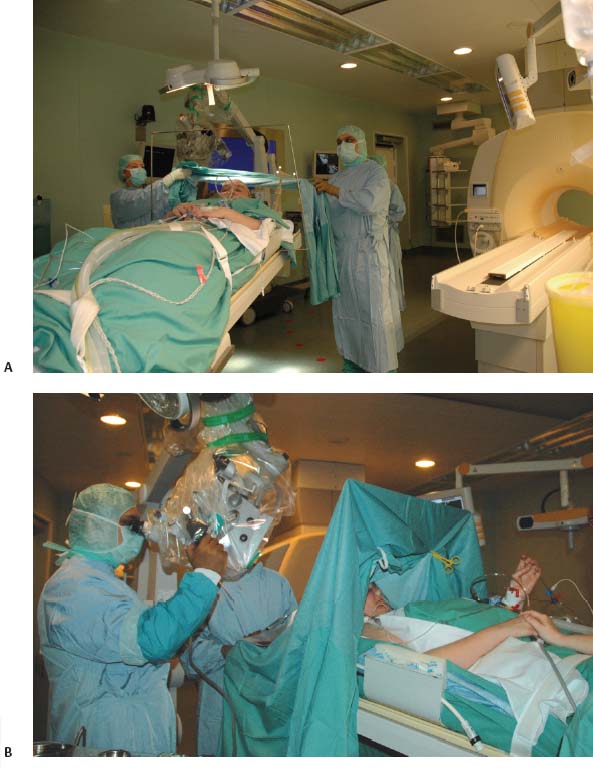
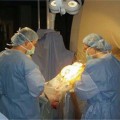
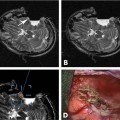
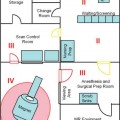
17 Throughout the development and implementation of intraoperative magnetic resonance imaging (iMRI), the goal has been to combine surgery and MRI1–6 such that MRI provides surgical guidance without impeding the use of state-of-the-art microneurosurgical techniques. The results of various groups have shown that iMRI provides the basis for more extensive glioma resection.1,4,7–11 We installed our customized integrated MRI operating room (MRI-OR) with a 1.5 tesla MRI scanner (Intera; Philips Healthcare, Andover, MA) in 2005. Our primary indications involve primary and recurrent gliomas (see Chapter 12). However, although most gliomas are situated in predominantly quiescent localizations and are thus potentially amenable to gross total volumetric resection, some lesions affect eloquent areas, in particular speech and motor cortex. These areas may be merely displaced by the lesion, but also infiltrated.12,13 Under the latter condition, gross total volumetric resection of the tumor is certain to cause unacceptable deficits affecting the patient’s quality of life. Although biopsy might be an option to define pathology, surgical resection and volume reduction remains the first step in the subsequent multimodal therapy. The challenge in these cases rests in the balance between the most complete surgical excision and conserving or preferably improving function, and hence quality of life. A thorough evaluation of such lesions has to combine structural as well as functional characteristics. Functional MRI (fMRI) is of great value in localizing areas at risk, either in the approach trajectory or the potential resection area. However, it does not yield certainty. It is well known that language production, comprehension, and association encompass multiple areas. Comprehensive investigation by imaging remains a challenge.14–16 Even for motor skills, which often show a good overlap of functional and anatomic representation on preoperative evaluation,17 direct motor responses may be elicited beyond the structural identifiable precentral gyrus.18 Acknowledging the incomplete representation of functional areas on preoperative imaging, we chose to gain additional information for surgical decision making. Therefore, we use direct cortical stimulation for electrophysiologic testing during “awake craniotomies”19–21 for patients with lesions in eloquent areas. This allows testing and monitoring of function, specifically speech and motor skills,22 and if necessary modification of surgical tactics. Since 1996, we have been acquiring experience with cortical stimulation during awake craniotomies. Building on this familiarity we transferred this technique to the setting of iMRI. In this chapter we describe our practice of combining iMRI and awake craniotomy. We installed a fully integrated iMRI-OR in our institution in 2005. At the core of this operating room is the short-bore 1.5 tesla MRI scanner (Intera; Philips Healthcare).4,7 The sequences are the same as in conventional MRI. We use flexible surface coils. Usually, we position one coil below the patient’s head, and for scanning the second coil is positioned on top of the head, opposite to the first one. Conventional microneurosurgery is performed outside of the 5 gauss (G) line, using ferromagnetic tools and equipment (such as the microscope, ultrasonic aspirator, bipolar coagulation, and cortical stimulation), as well as a ceiling-mounted navigation system (BrainLab VectorVision; BrainLab, Heimstetten, Germany). The patient is transferred from the microneurosurgical operation site to the scanner using a pivoting angiography table, which connects to the MRI. For this transfer the surgical site is covered with adhesive sterile draping. According to the MR characteristics of the lesion (e.g., enhancing or nonenhancing, T2 hyperintense, fluid attenuation inversion recovery [FLAIR] abnormality) the appropriate sequences are chosen. The imaging time is kept as concise as possible. Usually one neurosurgeon remains with the patient during scanning, which most patients consider very reassuring. The images are directly transferred to our navigation system via Ethernet. The patient is returned to the microneurosurgical operation site. For updated navigation we reattach the dynamic reference frame if further resection is necessary. Between September 2005 and October 2008, 348 surgeries were performed in this integrated MRI-OR suite. The main pathologies were gliomas (227) and pituitary lesions (52). In all, 209 glioma cases were limited to either hemisphere (117 left, 92 right). Thirty-four patients were operated on with cortical stimulation; four underwent repeat surgery for recurrent disease. Therefore, 38 awake craniotomy procedures were performed. Thirty-two cases had left-sided lesions, of which 14 were tested primarily for motor skills, and 18 for speech. Six patients with right-sided pathology underwent intraoperative monitoring, testing motor skills. Age ranged from 23 to 69 years (median age of 42 years). Initial patient selection was based on their neurologic symptoms and on the structural MRI. Patients harboring lesions within or abutting eloquent areas were considered for awake craniotomy. Mandatory workup included fMRI and neuropsychologic investigation. Awake craniotomy was considered, if the patient’s lesion extended into or infiltrated speech-associated areas: the left frontal operculum (for left dominant hemisphere) and the left dorsotemporal lobe. With regard to motor skill-associated areas, those lesions abutting or infiltrating the primary motor cortex, the left-sided supplementary motor area, and also the subcortical pathways were considered for awake craniotomy. Tumors within the right supplementary region, if the patient is clearly left dominant, are operated on under general anesthesia. Major neurologic deficits, which preclude intraoperative testing, are contraindications, such as pronounced aphasia, which would prevent speech testing. As for motor areas, unless the patient is unable to move at all, awake craniotomy should not be ruled out. After debulking and reduction of mass effect, the paresis might improve. However, if infiltration predominates, this might not happen, but worsening can be potentially avoided by discontinuing the operation. Subsequently, patients were evaluated by a neuropsychologist. Specific emphasis was given to potential compliance, anxiety, and intellectual capacity. Patients were excluded with a score below 23 points in the Mini Mental State Examination, and less than 50% in the examined language tests. Furthermore, inhibited, apathic, and disorganized behavior patterns were grounds to exclude patients because noncompliant behavior was likely (Goebel S, Nabavi A, Schubert S, et al. Patient perception of combined awake brain tumor surgery and intraoperative MRI. Accepted for publication in Neurosurgery). Naturally, claustrophobic patients cannot be considered for awake craniotomy in an iMRI. Having explained the rationale for potential awake craniotomy before testing the patients, we then discuss the test results and the surgical technique with the patients. We have found most patients to be motivated and compliant following this preoperative procedure. Only one patient declined due to psychological problems. We ascribe the high rate of acceptance of this technique to the thorough assessment prior to recommending the procedure. The anesthesiologist provides cardiovascular and pulmonary monitoring during surgery. Nerve blockades and local anesthesia of the prospective surgical area are done by the anesthesiologist and the neurosurgeon. The patient is positioned for surgery in a specially adapted MRI-compatible Mayfield clamp, rigidly attached to the MRI-table. Initial microneurosurgical tumor removal is guided by neuronavigation using preoperative imaging and cortical stimulation during neurophysiologic testing. On demand MRI is acquired and used for updated navigation. Throughout the procedure, low doses of analgesics are given. After resection and final testing, sedating medication is begun. The distinct steps are discussed in detail below. The anesthesiologist prepares the patient with intravenous (IV) and arterial lines, central venous catheter, and urethral catheter in a separate area for patient preparation. The occipital, temporal, and supraorbital nerves are blocked with depots of 2.5% Naropin (ropivacaine hydrochloride; AstraZeneca UK Ltd., London, UK). Then the patient is transferred to the iMRI-OR and placed on the table docked to the MRI. Positioning awake patients in an iMRI unit is even more of a challenge than in a conventional OR. Special care has to be taken to ascertain comfortable arrangements as well as access to the patient for intraoperative testing. In the iMRI, the mentioned goals have to be achieved, while having the further restraints of the MRI tunnel. Thus we position in the immediate vicinity of the MRI, to ensure the free passage into the bore for imaging. Although the head is rigidly fixed in the MRI-compatible Mayfield clamp, we place cushions beneath the neck. Patients have been observed to relax their neck muscles subsequently. It is important to ensure that the patient is as comfortable as possible. Small discomforts at the onset of surgery may result in total noncompliance toward the end of the resection. In addition, it should be noted that the table’s mount permits only raising and lowering of the head, tilting along the table axis is not possible. The patient’s head is rigidly fixed to the table with a specially modified carbon-fiber Mayfield clamp (Promedics GmbH, Dusseldorf, Germany). The projected pin insertion sites were infiltrated with 2 to 4 milliliters of 7.5% Naropin. More Naropin is injected as needed. One coil is permanently positioned below the patient’s head, within the Mayfield clamp, while the other surface coil is placed on top. This coil is removed during surgery, and repositioned for scanning (on top of the draping). After positioning we acquire a T2-weighted image (T2WI), to rule out artifacts. The patient is transferred to the surgical position; the navigation system is registered. The point of entry is defined using the navigation system, setting the best approach to the tumor. The flap is fashioned to provide enough space to perform cortical mapping and potentially use alternate routes to circumvent functional areas should they be encountered. The planned skin incision is infiltrated with 7.5% Naropin. If a straight incision is used, only the planned incision is infiltrated. For a skin flap, which is most frequently indicated, its base has to be infiltrated as well. The drapes are arranged in a tent-like fashion (Fig. 17.1) allowing access to the patient as well as visual contact, to allow testing (e.g., naming of drawn objects). Standard microneurosurgical techniques are employed. After placing the burr hole, additional Naropin-immersed cottonoids are placed on the dura. Throughout the opening additional doses of Ultiva (Remifentanil; Abbott Laboratories, Abbott Park, IL) are infused. Once the bone flap is turned, Naropin-immersed cottonoids are placed on the dura for local anesthesia. The dura is opened under the microscope. The brain itself is not pain-sensitive. However, the basal dura and larger vessels may elicit pain, when manipulated. Additional Naropin-immersed cottonoids are placed on the painful structures. Care must be taken that the anesthetic agent does not reach the cortical areas in question because that may hamper cortical stimulation. Employing neuronavigation, the best approach to the tumor is defined and its borders identified, respectively. Fig. 17.1 (A) Patient positioned for surgery in the intraoperative magnetic resonance imaging- operating room (iMRI-OR) suite. Drapes are arranged to allow access to the patient and visual contact. The pivoting angiography table is disconnected from the MRI scanner, and turned, to place the patient’s head into the “surgical area.” The monitor of the ceiling-mounted navigation system is visible on the right side. Draped microscope on the left in the background. (B) Position after draping. View from the other table side. MRI in the background. Motor testing during resection of a recurrent malignant glioma, extending from the right postcentral gyrus anteriorly into the pyramidal tract. The contrast-enhancing lesion was removed completely, the resection cavity lined with Gliadel wafers (MGI Pharma, Bloomington, MN). The patient had no motor deficit, perception of a “heavy leg,” as complained about prior to surgery, receded after tumor debulking. Various groups use propofol during craniotomy, and discontinue the drug before cortical stimulation. They have reported good results without adverse effect on cortical stimulation after the discontinuation of propofol.23,24 The neuropsychologist, typically the one who performed the preoperative evaluation, remains with the patient throughout the procedure. Testing is performed regularly as well as on the surgeon’s request (Fig. 17.1B). Throughout the surgery, the neuropsychologist and the surgeon speak with the patient. Cortical stimulation is established as a technique to identify functional cortical areas; subcortical stimulation helps to identify connective fiber tracts. It uses electrical current which, depending on the examined area, may elicit or impede responses. We use a bipolar cortical stimulator to investigate the cortex in the planned trajectory and for superficial lesions around the tumor. The cortex is touched in various areas (Fig. 17.2A, B,C), while the neuropsychologist tests the functions associated with that specific area – either speech or motor tasks. The basic elements of the tests were rehearsed prior to surgery. Speech monitoring is the most complex, ranging from naming to association and free narration. If functional areas are touched with the probe, disruption of the cortical electrical activity results in functional deficits. These are reversible with removal of the probe. If cortical stimulation results in reversible deficits, these areas may not be resected, even if obvious tumor infiltration is present.
Awake Craniotomy and Intraoperative MRI for the Resection of Gliomas
Preparation and Procedure
Intraoperative MRI-Operating Theater Set-up
Patients and Preoperative Evaluation
Overview of Procedure
Initial Arrangement, Positioning, and Commencing Surgery
Intraoperative Testing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree