Etiology, Prevalence, and Epidemiology
Pneumonia and influenza represent the eighth leading cause of mortality in the United States, with an estimated 1.3 million hospital admissions yearly attributable to pneumonia in patients older than 65 years. Although pneumonia affects all ages, increased morbidity and mortality are seen at the extremes of age (very young and very old).
Pneumonia is broadly divided into infection acquired in the community versus acquired in health care settings, including hospitals and nursing homes. Multidrug-resistant (MDR) bacteria are more commonly encountered in hospital or health care–associated pneumonia.
Community-acquired pneumonia (CAP) manifests in a patient who has not been recently hospitalized and who has had no regular exposure to the health care system. Incidence in the general population is approximately 5 to 6 per 1000 person years, with about 5.6 million cases of CAP encountered per year, representing one of the most commonly treated infectious diseases. Pneumococcus remains the most common bacterial pathogen responsible for CAP, although its incidence has declined with the widespread use of the pneumococcal vaccination and with decreased rates of cigarette smoking. In the late 1930s a separate clinical course was described for pneumonia with a longer more indolent onset that progressed to the more typical clinical presentation. This form was termed atypical , and the causative agents were termed atypical infections. In addition, atypical pneumonia often is associated with more systemic manifestations of disease, including gastrointestinal abnormalities, hepatitis, and meningoencephalitis. With patients surviving longer, this atypical presentation is now seen with both the traditional atypical organisms as well as other infectious agents, making the distinction imprecise and anachronistic. However, given its widespread use in clinical practice, the grouping of typical and atypical causes of pneumonia has persisted. Typical organisms causing CAP include Streptococcus pneumoniae , Haemophilus influenzae , Staphylococcus aureus , group A streptococci, Moraxella catarrhalis , anaerobes, and aerobic gram-negative bacteria. Among the atypical infections are Legionella spp., Mycoplasma pneumoniae , and Chlamydia pneumoniae .
Health care–associated pneumonia (HCAP) occurs in patients who have recently been hospitalized for 2 or more days in the 90 days preceding infection; resided in a nursing home or other long-term–care facility; or received recent intravenous antibiotic therapy, chemotherapy, hemodialysis, or wound care within the 30 days preceding infection. Patients with HCAP are more commonly infected with antibiotic-resistant bacteria, such as methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa, and Acinetobacter spp.
Hospital-acquired pneumonia (HAP) is defined as pneumonia occurring 48 hours or more after a hospital admission. Ventilator-associated pneumonia (VAP) manifests 48 to 72 hours after endotracheal intubation. HAP and VAP combine to represent the most common cause of death among all hospital-acquired infections, with mortality rates ranging from 20% to 50%. Common pathogens for both HAP and VAP include gram-negative bacilli (e.g., Escherichia coli , Klebsiella pneumoniae , Enterobacter spp., Pseudomonas aeruginosa , Acinetobacter spp.), and gram-positive cocci (e.g., S. aureus , including MRSA, and Streptococcus spp.). Specifically, with VAP, patients are also commonly found to be infected with oral anaerobic bacteria.
Clinical Presentation
Bacterial pneumonia may have a rapid onset with classic clinical symptoms consisting of high fever, productive cough, sweats, and chills. Some patient groups, particularly the elderly, may demonstrate a more gradual course with less severe symptoms. With infection by some atypical organisms, such as Legionella and Mycoplasma, systemic symptoms are common. These can include diarrhea, myalgias, headache and confusion, anemia, or skin rash.
Pathologic Findings and Pathophysiology
Particulate material and microbes within the upper respiratory tree, either aerosolized particles or oral secretions, may enter the lower respiratory tract through microaspiration. Even so, lower respiratory tract defenses can typically maintain a sterile environment unless there is a defect in the host defenses, exposure to a particularly virulent organism or overwhelming inoculum. Less commonly, infection can occur via hematogenous spread from bacteremia, infective endocarditis, or infected catheters. Direct spread of infection is uncommon but can arise from infections of the mediastinum or from adjacent organs, such as a liver abscess or esophageal perforation.
Pathologic findings depend upon the pattern of infection; lobar pneumonia, bronchopneumonia, interstitial pneumonia and bronchiolitis, or hematogenous spread of infection.
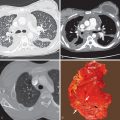
Lobar pneumonia is characterized histologically with filling of the alveoli by exudative fluid and neutrophils. The consolidation typically starts at the periphery of the lung and spreads centripetally via the pores of Kohn and small airways. This airspace filling will extend across pulmonary segments, sometimes involving the entire lobe ( Fig. 9.1 ).

Bronchopneumonia typically shows inflammatory exudate of polymorphonuclear leukocytes within the respiratory bronchioles. This exudate and the associated terminal bronchiolitis likely limits spread of infection initially, resulting in the characteristic patchy appearance seen on radiography ( Fig. 9.2 ). Bronchopneumonia may be associated with areas of parenchymal destruction or necrosis.

Interstitial pneumonia and bronchiolitis are characterized by inflammatory infiltrate within the bronchiolar wall with associated neutrophil-rich intraluminal exudate. Extension into the lung parenchyma results in segmental areas of consolidation. Interstitial pneumonia is more commonly associated with atypical organisms, such as Mycoplasma, viruses, or Pneumocystis jirovecii .
Hematogenous spread of infection (septic emboli) occurs secondary to friable thrombus either along a cardiac valve, bacteremia, or infected catheter or pacemaker wire that breaks off because of turbulent flow and lodges in a pulmonary capillary bed. This form of infection is most commonly associated with S. aureus . An uncommon source of septic emboli is thrombophlebitis of the internal or external jugular vein secondary to oropharyngeal infection with Fusobacterium necrophorum , referred to as Lemierre syndrome.
Inflammatory changes from pneumonia may irritate the adjacent pleural lining, resulting in increased capillary permeability and an exudative pleural effusion ( Fig. 9.3 ). Histochemically, this “simple” parapneumonic effusion has normal pH and glucose. If this is left untreated, the effusion may progress to a fibropurulent empyema.

Imaging Findings
Radiography and Computed Tomography
Imaging findings of bacterial pneumonia depend upon the pattern of infection, with certain organisms favoring certain patterns, which may suggest a class of organism, although no sign or pattern is specific.
Lobar Pneumonia
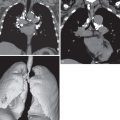
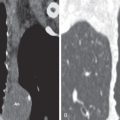
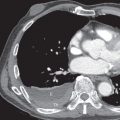
Lobar pneumonia is characterized by homogeneous airspace consolidation involving adjacent segments of a lobe ( Fig. 9.4 ; see Fig. 9.1 ), often bounded by an interlobar fissure ( Fig. 9.5 ) or the hemidiaphragm. The consolidation tends to occur initially in the peripheral subpleural lung and spreads centrally and may eventually involve the entire lobe, although this is uncommon with early initiation of antibiotics. Air bronchograms are nearly universally present within the consolidation because of the exudative process involving the alveoli rather than the bronchioles ( Fig. 9.6 ). On computed tomography (CT) ground-glass opacities adjacent to the consolidation represent incomplete alveolar filling ( Fig. 9.7 ).




Classic signs associated with lobar pneumonia include the “spine sign” with paradoxical increased density of the lower thoracic spine on the lateral radiograph resulting from the overlying lower lobe consolidation ( Fig. 9.8 ). Uncommonly encountered, the “bulging fissure sign” is due to copious amounts of exudative material within the consolidation, resulting in bulging or displacement of the fissures ( Fig. 9.9 ). Although classically described with K. pneumoniae , the bulging fissure sign is more commonly seen with S. pneumoniae given its greater prevalence.


Lobar pneumonia is most commonly seen with infection by S. pneumoniae , K. pneumoniae , and L. pneumophila .
Bronchopneumonia and Bronchiolitis
Bronchopneumonia begins with infection of the respiratory bronchioles, eventually extending into the adjacent alveoli. This typically manifests as poorly defined centrilobular or airspace nodules measuring 5 to 10 mm in diameter, tree-in-bud opacities reflecting the alveolar and bronchiole involvement ( Fig. 9.10 ; see Fig. 9.2 ), and patchy areas of consolidation often involving multiple segments of multiple lobes ( Fig. 9.11 ). Confluence of these areas of pneumonia in adjacent lobar segments may mimic a lobar pneumonia pattern. Distinction may be inferred by noting the segmental distribution of abnormalities in other areas.


Because of the tissue destruction and necrosis often associated with bronchopneumonia, areas of cavitation are commonly encountered, especially in patients with extensive consolidation. The airway involvement can result in volume loss within the affected segments or lobes. Air bronchograms are rarely seen secondary to the intraluminal inflammatory exudate within the bronchi and bronchioles.
With widespread bronchial wall inflammation manifesting as bronchial wall thickening and resultant luminal narrowing, mosaic attenuation of the lung parenchyma can often be seen representing air-trapping.
Bronchopneumonia is most common with infection by S. aureus , gram-negative organisms, anaerobic bacteria, and L. pneumophila . When only the interstitial manifestations of infection are encountered, including bronchiolitis, bronchial wall thickening, and endoluminal mucous plugging, atypical organisms such as Mycoplasma, viruses, or Pneumocystis jirovecii are more commonly the infectious agents.
Hematogenous Spread of Infection
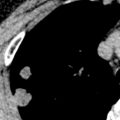
Hematogenous spread of bacterial infection (septic emboli) most commonly occurs in intravenous drug users or in patients with infected central catheters. The most common manifestation is multiple middle and lower lung zone poorly defined nodules and wedge-shaped consolidations, frequently demonstrating cavitation on both radiography and CT ( Figs. 9.12 and 9.13 ). This apicobasal gradient reflects the preferential blood flow within the lower, dependent portions of the lungs.


These nodules often have closely associated pulmonary vasculature termed the feeding vessel sign . Although these vessels may appear to lead to the nodule, thin-section CT imaging has shown that the feeding vessel is actually a pulmonary vein coursing from the nodule, whereas the pulmonary artery actually courses around the nodule ( Fig. 9.14 ). Occlusion of the pulmonary arteries with septic emboli may result in hemorrhage and/or infarction, resulting in ill-defined wedge-shaped areas of consolidation or ground-glass opacities (see Fig. 9.13 ).

Hematogenous spread of infection is most commonly seen with infections caused by Staphylococcus spp., usually S. aureus . As mentioned earlier, multiple septic emboli may be encountered less commonly in patients with thrombophlebitis of either the internal or external jugular vein termed Lemierre syndrome ( Fig. 9.15 ).

Complications
Especially with long-standing untreated pneumonia, complications including lung abscess formation or lung necrosis, empyema, or chest wall invasion can be seen and must be recognized as they can affect treatment and prognosis.
On contrast-enhanced CT in a patient with pneumonia, areas of inhomogeneous enhancement or cavitation are suggestive of lung necrosis. Lung abscesses usually manifest as thick-walled, peripherally enhancing fluid collections, commonly associated with an air-fluid level ( Fig. 9.16 ). This air-fluid level is similar in length on both posteroanterior and lateral projections owing to the spherical shape of the abscess ( Fig. 9.17 ). This is in contradistinction to empyema, which is lenticular in shape and therefore shows different lengths of the air-fluid level on orthogonal projections ( Fig. 9.18 ). The internal margin of the abscess wall is predominantly smooth (approximately 90% of cases), and the abscess has adjacent parenchymal consolidation in 50% of cases.



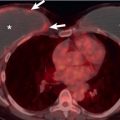
Usually manifesting as a small to moderate pleural effusion, a parapneumonic effusion represents free-flowing exudative fluid secondary to increased capillary permeability from irritation by the adjacent pneumonia. As this effusion progresses, internal fibrous septa develop, resulting in loculation and empyema formation. The “split pleura sign” is specific for an exudative effusion and is seen in 50% of all empyemas. This sign represents thickening and enhancement of the visceral and parietal pleura, separated by the loculated fluid ( Fig. 9.19 ). Presence of air within the pleural fluid collection in the absence of recent intervention should be regarded as diagnostic of a bronchopleural fistula until proven otherwise ( Fig. 9.20 ).


Although more commonly associated with certain fungal infections, such as blastomycosis, aspergillosis, and mucormycosis, direct invasion of the chest wall or hemidiaphragm may be seen with aggressive bacterial infections from actinomycosis, Nocardia, and mycobacterial organisms.
Pneumatoceles manifest as thin-walled, gas-filled spaces within areas of consolidation or ground-glass opacities ( Fig. 9.21 ). A clearly defined thin wall differentiates pneumatoceles from cavitation and lung abscesses. Pneumatoceles demonstrate a typical course with enlargement over days to weeks with resolution over weeks to months. Occasionally, pneumatoceles may rupture, causing a pneumothorax ( Fig. 9.22 ) or pneumomediastinum. Pneumatoceles occur most commonly in S. aureus and P. jirovecii pneumonia but also may be seen in other infections, including pneumococcal pneumonia.


Specific Organisms
Despite in-depth evaluation, including cultures and polymerase chain reaction testing, only 20% to 50% of cases of pneumonia in adults have a causative organism identified. This is likely due to early administration of antibiotic therapy and the less severe symptoms in the elderly.
Community-Acquired Pneumonia
Streptococcus pneumoniae
S. pneumoniae is a gram-positive bacterium, commonly associated with CAP. Currently, S. pneumoniae is detected in about 6% of cases of CAP overall, although it accounts for 20% to 60% of cases in older adults. Historically, pneumococcal pneumonia was determined to be the causative organism in 80% of cases of CAP, with the decrease likely due to widespread use of the pneumococcal vaccine in both children and older adults. Infection with S. pneumoniae is associated with chronic heart or lung disease, alcoholism, institutionalization, and prior splenectomy.
S. pneumoniae characteristically manifests as a lobar consolidation, involving the peripheral lung and abutting the visceral pleura ( Fig. 9.23 ). Occasionally, infection may manifest as a spherical focus of consolidation that simulates a mass (round pneumonia), although this pattern is seen more commonly in children than in adults ( Fig. 9.24 ). Although a lobar pneumonia pattern is the most frequent manifestation, given how common it is as an infectious agent, other patterns of infection, such as bronchopneumonia, will also be seen.


Pleural effusion is evident in about 10% of patients overall ; it affects 30% of patients who have severe pneumonia requiring treatment in the intensive care unit and 50% of patients with bacteremia. Lymphadenopathy is seldom apparent on the radiograph but is evident on CT in 50% of cases.
Most patients respond rapidly to antibiotic therapy. If, despite therapy, patients with clinical signs of pneumococcal pneumonia develop complications, such as lung abscess or necrosis, microaerophilic streptococcal infection, representing aspiration of oral flora, should be considered.
Haemophilus influenzae
H. influenzae is a gram-negative coccobacillus, accounting for 5% to 20% of CAP in patients in whom an organism can be identified successfully. Widespread administration of H. influenzae type B vaccine in children has nearly eliminated this subtype as an infectious agent in pneumonia. Nontypeable H. influenzae is the most common bacterial cause of acute exacerbation of chronic obstructive pulmonary disease (COPD).
Clinically, pneumonia caused by H. influenzae is indistinguishable from other bacterial pneumonias. Radiographically, H. influenzae pneumonia may manifest with either a lobar or bronchopneumonia pattern ( Fig. 9.25 ). Nodular opacities, by themselves or in combination with airspace consolidation, can also be seen, reflecting the presence of cellular bronchiolitis. On CT H. influenzae may occasionally demonstrate a diffuse micronodular pattern with bilateral centrilobular nodules measuring less than 5 mm in diameter. Pleural effusion may be seen in up to 50% of patients, although empyema is rare.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree