Congenital lung disease remains a confusing topic owing to ever-changing histopathologic understanding of the various entities and frequent updates to antiquated clinical and pathologic classification schemes. Past classifications have been based on thoracic anatomic structures, dividing lesions into congenital malformations of the trachea, bronchi, lung, and pulmonary vasculature. Such divisions are controversial because the embryologic basis of these malformations is not clearly understood, as more recent investigations have demonstrated significant overlap or coexistence of histopathologic features among these entities. The current literature points instead to a spectrum of congenital bronchopulmonary malformations (CBM) rather than separate individual entities, which classically included congenital pulmonary airway malformation (CPAM) (formerly congenital cystic adenomatoid malformation [CCAM]), intralobar and extralobar sequestration, and foregut cysts. All exhibit some failure of budding, differentiation, or separation from the primitive foregut between the 3rd and 24th weeks of gestation, with or without superimposed airway obstruction.
Most of these entities are now diagnosed with prenatal imaging and regress prior to birth. However, some can remain asymptomatic during childhood and are diagnosed in the adolescent or adult. This leads to controversy over perinatal management. In select cases, in utero or early postnatal surgery may be required for large lesions that contribute to significant fetal compromise. In this chapter we limit our discussion to developmental abnormalities that are discovered in adults. Diagnosis of most CBMs can be achieved with radiography, prenatal ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). Management of CBMs is variable, ranging from conservative observation to surgical excision, and is usually dependent on symptomatology or the potential risk of complications.
Anomalous Tracheobronchial Branching
Tracheal Bronchus
Etiology, Prevalence, and Epidemiology
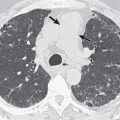
Variable branching patterns of the tracheobronchial tree have been well described. When an anomalous bronchus arises from the right lateral wall of the trachea (usually <2 cm from the carina) and supplies all or segments of the right upper lobe, it is referred to as a tracheal bronchus. However, various other authors have used the term tracheal to describe any anomalous bronchus derived from the trachea and main bronchus supplying an upper lobe. The anomalous tracheal bronchus is referred to as supernumerary when the segmental bronchi to the upper lobes exhibit a standard branching pattern—three to the right and four to the left upper lobes. In the absence of one or more of the normal upper lobe segmental bronchi, the tracheal bronchus is considered as displaced. The term pig bronchus (bronchus suis) is applied when the entire right upper lobe is supplied by the tracheal bronchus. When a tracheal bronchus ends blindly, it is called a tracheal diverticulum. The territory ventilated by the anomalous bronchus usually has normal vascular supply.
The reported prevalence of a right tracheal bronchus ranges from 0.1% to 2%. The reported frequency of a tracheal bronchus supplying the entire right upper lobe (pig bronchus) is approximately 0.2%. Rarely, a similar malformation may be seen on the left side. However, most cases were bilateral tracheal bronchi or associated with bilateral right tracheal bronchi in the setting of right isomerism (described subsequently).
Clinical Presentation
The vast majority of adult patients with a tracheal bronchus are asymptomatic, and the abnormal bronchus is discovered incidentally on CT or at bronchoscopy. The anomaly may become clinically significant when the inhibited clearance of the anomalous bronchi leads to conditions such as recurrent right upper lobe pneumonia, air trapping, lobar or segmental postobstructive atelectasis, or bronchiectasis.
Manifestations of the Disease
Computed Tomography.
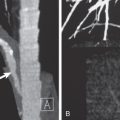
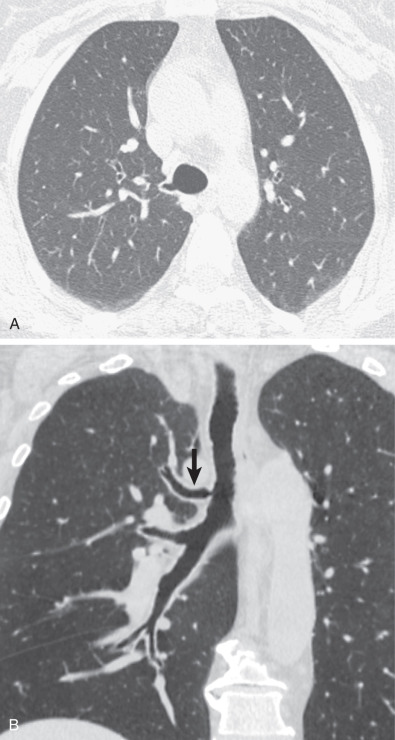
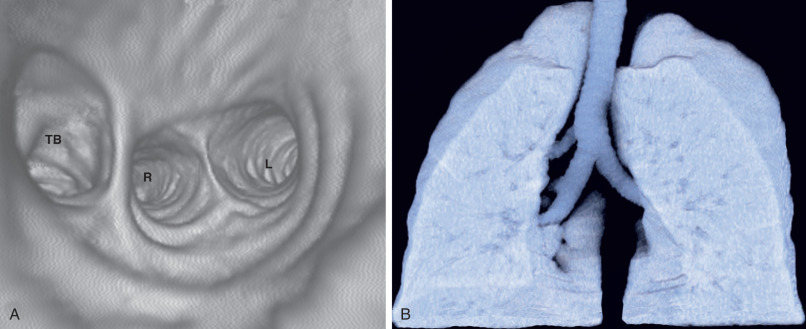
Tracheal bronchi, as well as the presence of associated findings such as bronchiectasis or emphysema, are best characterized by CT ( Fig. 7.1 ). Multiplanar reformations, volume-rendered imaging, and virtual bronchoscopy techniques allow reconstruction of CT data to produce three-dimensional images of the tracheobronchial tree ( Figs. 7.1 and 7.2 ).
Synopsis of Treatment Options
Treatment of symptomatic patients is based on the severity of symptoms and ranges from observation to lobectomy.
Accessory Cardiac Bronchus
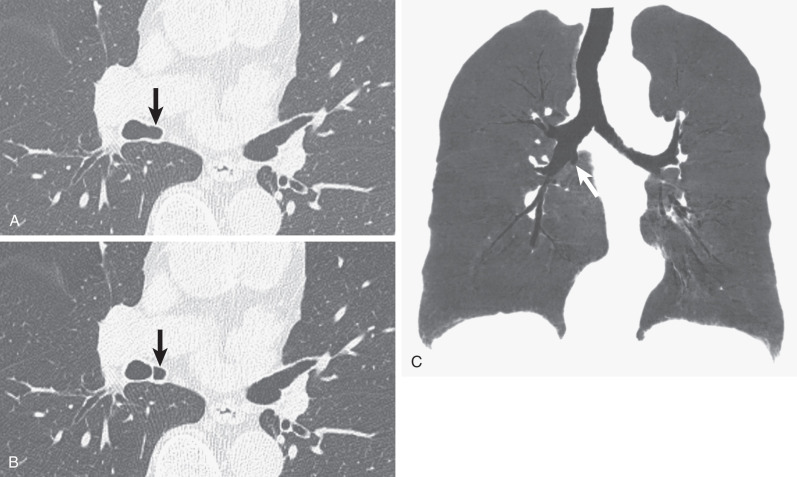
An accessory cardiac bronchus (ACB) is a mucosa-lined supernumerary bronchus that arises from the medial wall of the right main bronchus or bronchus intermedius. The bronchus courses medially and caudally for 1 to 5 cm. The presence of mural cartilage distinguishes ACB from a diverticulum. ACBs usually end blindly but may be associated with small amounts of abnormal pulmonary parenchyma (a so-called cardiac lobe). The anomaly occurs in up to 0.5% of the general population.
Clinical Presentation
The vast majority of adult patients are asymptomatic. The most common complications are cough and hemoptysis secondary to infection or rarely carcinoma. Pneumothorax may result from traumatic injury to the ACB during bronchoscopy.
Manifestations of the Disease
Computed Tomography.
The diagnosis of an accessory cardiac bronchus is best accomplished with CT. As with other bronchial anomalies, the course of the ACB is best depicted with reformation techniques, such as multiplanar reconstruction, volume rendering, or virtual bronchoscopy ( Fig. 7.3 ).
Synopsis of Treatment Options
Treatment of symptomatic patients is based on the severity of symptoms and ranges from observation to surgical resection.
Displaced Bronchi
Approximately 10% of individuals have displaced segmental or subsegmental bronchi in the same lobe, which are best recognized on CT. Less commonly, a segmental or subsegmental bronchus originates from an adjacent lobe. The lung ventilated by the displaced bronchus is normal, but CT often demonstrates an incomplete fissure between the adjacent lobes. Occasionally, segmental bronchi may be absent (bronchial agenesis). Segmental bronchial agenesis occurs most commonly in the right upper lobe. Displaced segmental and subsegmental bronchi are asymptomatic and are found incidentally on CT or at bronchoscopy.
Congenital Bronchial Atresia
Etiology, Prevalence, and Epidemiology
Bronchial atresia occurs when a lobar, segmental, or subsegmental bronchus is congenitally truncated at or near its origin. The bronchi and alveoli distal to the obstruction develop normally. The pathogenesis of bronchial atresia is unknown but proposed to either represent sequelae of an ischemic event to the developing bronchial wall or a developmental error wherein the bronchial lung bud separates from the more distal cell mass of primitive bronchi. Because of its frequent association with other congenital bronchopulmonary malformations (sequestration, CPAM, and congenital lobar overinflation), bronchial atresia is believed to share a common pathogenic mechanism among these entities. Bronchial atresia most commonly occurs in the apicoposterior left upper lobe bronchus, followed in order of frequency by segmental bronchi of the right upper lobe and less commonly the middle lobe and the lower lobes. The estimated population prevalence is approximately 1 case per 100,000 persons and is more common in males.
Clinical Presentation
The majority of patients are asymptomatic. Some have recurrent pneumonia.
Pathophysiology
Pathologically, the bronchial tree distal to the point of obliteration is patent and has a normal number of airways and airspaces. A mucocele develops in the distal bronchi, which become dilated and filled with mucus that cannot be cleared through the disconnected larger airway. Because air can enter the affected bronchopulmonary segments only via collateral channels, overinflation and expiratory air trapping within the distal alveoli result.
Manifestations of the Disease
Radiography
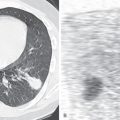
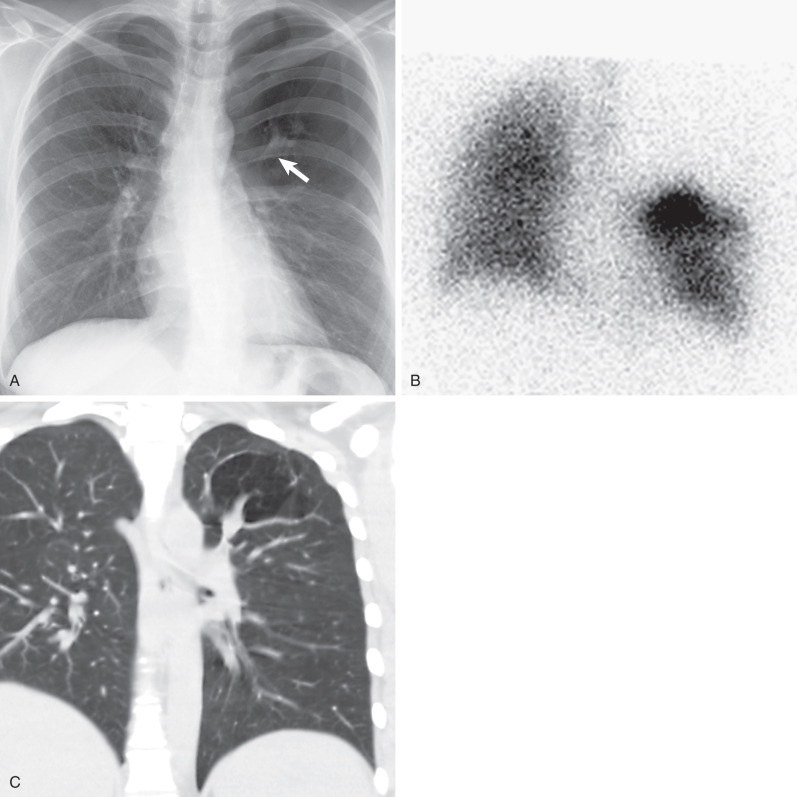
The radiographic findings are usually characteristic and consist of an area of pulmonary hyperlucency, seen in approximately 90% of cases, and a hilar nodule or mass, seen in 80% ( Fig. 7.4 ). The hyperlucency results from a combination of oligemia and an increase in the volume of air within the affected parenchyma. The adjacent normal lung is compressed and displaced; the mediastinum may or may not show displacement. Accumulation of secretions and mucoid impaction distal to the bronchial atresia result in ovoid, round, or branching opacities near the hilum. Atelectasis may result in adjacent lung segments as a result of compression and mass effect from the hyperinflated lung tissue.
Computed Tomography
Excellent visualization of the mucoid impaction and segmental overinflation and hypovascularity is afforded by CT. Mucoid impaction is readily recognized by the presence of branching soft tissue densities in a bronchial distribution, usually associated with bronchial dilation. Bronchial occlusion, mucoid impaction with bronchial dilation (mucocele) immediately distal to the atretic bronchus, as well as decreased vascularity and attenuation and increased volume of the affected segment are evident on CT (see Fig. 7.4 ). Expiratory CT will demonstrate air trapping in the affected lung segment.
Magnetic Resonance Imaging
The diagnosis of bronchial atresia can be made on MRI. Mucoid impaction has variable signal intensity on T1-weighted images but typically very high signal intensity on T2-weighted images.
Imaging Algorithm
Although the radiographic findings may be characteristic, CT is the most sensitive imaging technique for confirming the diagnosis. It allows excellent visualization of the mucoid impaction, segmental hyperlucency, and decreased vascularity, a combination of findings that is generally considered diagnostic. Mucoid impaction is readily recognized by the presence of branching soft tissue densities in a bronchial distribution, usually associated with bronchial dilation. Although a similar appearance has been reported on MRI, CT is the imaging modality of choice.
Differential Diagnosis
The main differential diagnosis is partial airway obstruction caused by a foreign body, endobronchial tumor, or acquired bronchocele (e.g., allergic bronchopulmonary aspergillosis or bronchiectasis). Similar to these conditions, the lung distal to bronchial atresia is hyperlucent and shows air trapping. However, endobronchial lesions are rarely, if ever, associated with overinflation of affected bronchopulmonary segments at total lung capacity, an almost invariable finding in bronchial atresia. Therefore the distinction can often be made on a chest radiograph performed at maximal inspiration. A confident diagnosis of mucoid impaction distal to bronchial atresia can generally be made on CT. However, bronchoscopy may be required to rule out other causes of obstruction.
Synopsis of Treatment Options
Treatment may consist of conservative management in asymptomatic patients. However, some authors advocate for surgical resection of bronchial atresia, even in asymptomatic patients, given the inherent risk for recurrent infection.
- •
Most commonly involves the left upper lobe apicoposterior segmental bronchus
- •
Common radiologic findings:
- •
Chest radiography typically shows hyperlucency (from collateral ventilation and air trapping) of the affected segment, decreased vascularity, and a juxtahilar nodule or mass.
- •
CT shows a round, oval, or branching central mucoid impaction, segmental overinflation, and hypovascularity.
- •
Impacted mucus exhibits high signal intensity on T2-weighted MRI.
- •
Bronchopulmonary Isomerism and Heterotaxy
Thoracoabdominal visceral relationships are described in terms of situs, depending on the position of the cardiac atria and abdominal viscera relative to the midline. Situs solitus, situs inversus, and situs ambiguous (so-called heterotaxy) have been used to describe the normal, mirror image of normal, and non–mirror-image deviation from the normal left-right visceroatrial configuration, respectively. Visceroatrial situs should include a description of the position of the upper lobe bronchi to their ipsilateral pulmonary arteries—the most reliable imaging feature of bronchial situs, and thus atrial morphology. The morphologic right lung is defined when the upper lobe bronchus is positioned above the ipsilateral pulmonary artery in the hilum (eparterial). The morphologic left lung is supplied by a hyparterial bronchus, which courses below the ipsilateral pulmonary artery.
Heterotaxy syndromes are frequently associated with bronchopulmonary isomerism—an identical pattern of branching and lobar configuration in each lung. Bilateral eparterial and hyparterial bronchi characterize right and left bronchial isomerism (bilateral right- and left-sidedness), respectively. Right isomerism (bilateral right-sidedness) typically manifests with bilateral trilobed lungs and eparterial bronchi, bilateral right atria, right aortic arch, and asplenia (or some functional loss). Right isomerism is commonly associated with severe congenital heart disease with high mortality rates. Left isomerism typically manifests with bilateral bilobed lungs and hyparterial bronchi, bilateral left atria, and polysplenia (although this feature is variable). Other commonly associated (although variable) findings include midline liver and intestinal malrotation in both forms of heterotaxy. Left isomerism may also manifest with azygos or hemiazygos continuation of the inferior vena cava (IVC) and a truncated pancreas.
Etiology, Prevalence, and Epidemiology
Heterotaxy syndrome is relatively uncommon, occurring in 1 of 10,000 births. It is difficult to establish the exact prevalence of left isomerism, as patients without symptomatic congenital heart disease may be incidentally diagnosed in adulthood. Situs inversus has a prevalence of 0.01% of the population. Although likely multifactorial, the etiology of isomerism is probably related to defects in various genes (e.g., ZIC3 , Pitx-2, Cited-2 , Sonic hedgehog, Lefty-1 ) that regulate right- or left-sided patterns during early embryogenesis. Furthermore, isomerism is associated with other defects included as part of the spectrum of ciliopathies (e.g., immotile cilia syndrome, sinonasal abnormalities, bronchiectasis, biliary atresia, and brain malformations).
Clinical Presentation
Symptoms of bronchopulmonary isomerism are dependent on the severity of associated abnormalities and may range from intractable wheezing and infantile heart murmurs to cyanosis and heart failure. More common in males, right isomerism confers a poorer prognosis and frequent association with asplenia (leading to immune compromise and sepsis) and severe cyanotic congenital heart lesions (e.g., common atrioventricular canal, monoventricle, transposition of the great arteries, and total anomalous pulmonary venous return). Left isomerism (bilateral left-sidedness) is often associated with polysplenia, and although it has an overall better prognosis compared with right isomerism, there is still a 60% mortality in the first year of life. Congenital heart defects (most commonly partial anomalous pulmonary venous return, atrial septal defect, and atrioventricular canal) are less frequent or less severe compared with those of right isomerism, but patients may still present with congestive heart failure from left-to-right shunts. Patients may also present with midgut volvulus related to intestinal malrotation.
Manifestations of the Disease
Ultrasound
Situs anomalies may be diagnosed on prenatal ultrasound and suggested by the right-sided location of the aorta, cardiac apex, and stomach bubble (inversus) or some deviation thereof, raising suspicion of heterotaxy. Ultrasound can also detect variable venous drainage patterns in the setting of anomalous pulmonary venous return, and the position of the atria may be implied by infradiaphragmatic venous drainage patterns. Abdominal ultrasound in neonates may quickly and safely document polysplenia or asplenia.
Radiography
Historically, chest radiography was used to determine the position of the cardiac apex and stomach bubble but was inconsistent. Furthermore, the presence of bilateral horizontal fissures or eparterial bronchi may be detected on frontal chest radiographs, suggesting right isomerism, or bilateral hyparterial bronchi in the setting of left isomerism. A right-sided aortic arch in the setting of right isomerism and azygos continuation of the IVC in left isomerism can both be detected on chest radiography. Chest radiography may also detect the presence of anomalous pulmonary vessels in the setting of anomalous pulmonary venous return.
Computed Tomography
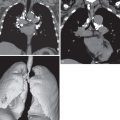
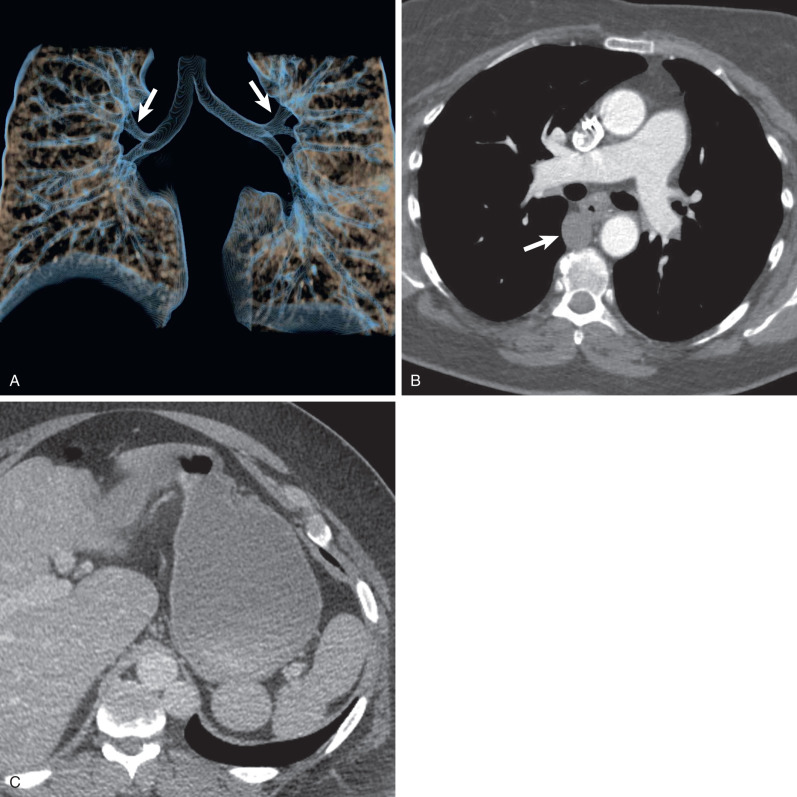
Today, radiography may identify the presence of situs ambiguous, but cross-sectional imaging is necessary for more detailed categorization of the associated anatomic anomalies. CT of the chest, abdomen, and pelvis readily characterizes and details situs and associated anatomic anomalies ( Fig. 7.5 ). Contrast-enhanced CT allows assessment of cardiac and vascular structures in the setting of complex congenital heart defects and anomalous drainage. CT and fluoroscopy may also detect the site of volvulus in complicated intestinal malrotation.
Magnetic Resonance Imaging
MRI demonstrates exquisite cardiac detail and is frequently used in the evaluation of congenital heart disease, particularly in identifying atrial morphology. In may be the preferred modality for evaluation of children given the risk of radiation exposure from CT.
Differential Diagnosis
Findings in heterotaxy syndrome are variably present, and thus the differential diagnosis would include situs solitus with congenital heart disease (0.6%–0.8%), right aortic arch, situs solitus with malrotation, hypogenetic lung (scimitar) syndrome, primary ciliary dyskinesia, and Kartagener syndrome.
Synopsis of Treatment Options
Treatment is determined by the severity and type of malformation present. Typically, early- and often-staged surgical intervention is used for severe cyanotic congenital heart disease.
- •
Situs solitus, situs inversus, and situs ambiguous (so-called heterotaxy) describe the thoracoabdominal visceral relationships, depending on the position of the cardiac atria and abdominal viscera relative to the midline.
- •
Bilateral eparterial or hyparterial bronchi (bronchial situs) is the most reliable predictor of atrial situs.
- •
Both right and left isomerisms have a strong association with congenital heart defects and intestinal malrotation, and more severe defects (more common with right isomerism) portend a poor prognosis.
- •
Common radiologic findings:
- •
Radiographs may detect discordant positioning of the cardiac apex and gastric bubble, azygos continuation of the IVC, or anomalies of pulmonary venous drainage, which may raise suspicion for underlying heterotaxy.
- •
CT and MR are frequently indicated to better characterize underlying associated visceral anomalies.
- •
Bronchogenic Cyst
Etiology, Prevalence, and Epidemiology
Bronchogenic cysts result from abnormal separation of localized portions of the tracheobronchial tree from the adjacent airways between the 3rd and 24th weeks of gestation. Most are isolated lesions but may be found in association with other congenital bronchopulmonary malformations, such as sequestration. Approximately 75% to 90% are located in the mediastinum. Other locations include the lung parenchyma, pleura, diaphragm, neck, or abdomen.
Clinical Presentation
The majority of bronchogenic cysts are asymptomatic and found incidentally on a chest radiograph or CT. Symptoms are most frequently caused by compression of the trachea or bronchi, producing cough, wheezing, stridor, and pneumonia. Less commonly, bronchogenic cysts may compress the esophagus and lead to dysphagia or compress an adjacent pulmonary vein and result in localized pulmonary edema. Infection occurs in approximately 20% of patients with intraparenchymal cysts. Less common manifestations of intraparenchymal cysts include hemoptysis and pneumothorax.
Pathophysiology
Bronchogenic cysts are thin walled, unilocular, and roughly spherical in shape. They are filled with serous or mucoid fluid. The cyst wall is lined by respiratory epithelium and contains smooth muscle and cartilage. Calcification may occur in the cyst wall or within the mucoid contents. Although bronchogenic cysts do not initially communicate with the tracheobronchial tree, communication may occur secondary to instrumentation or infection.
Manifestations of the Disease
Radiography
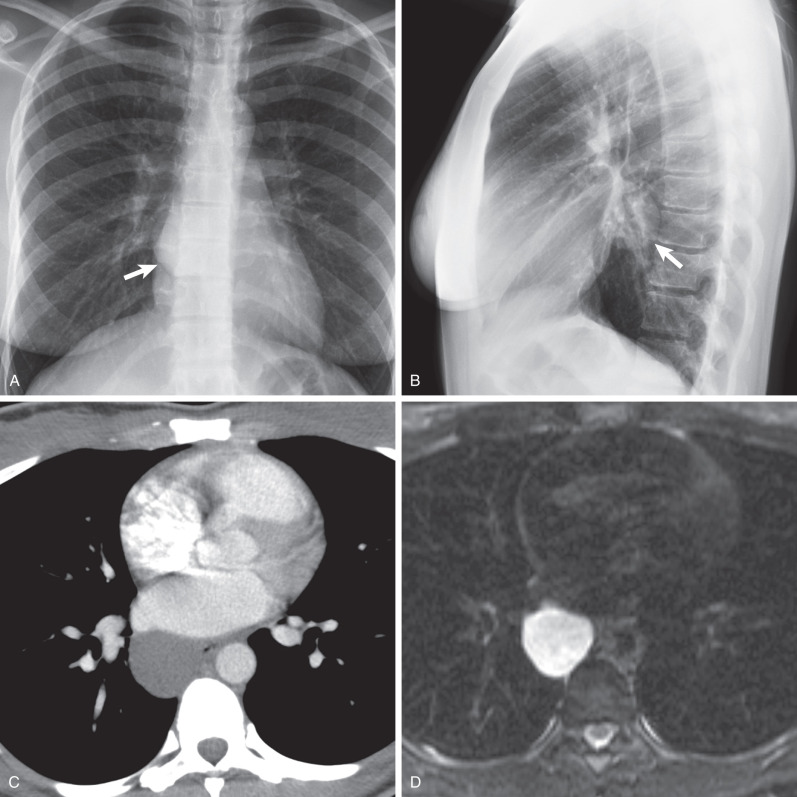
Radiographically, mediastinal bronchogenic cysts are usually seen as round or oval mediastinal masses in the subcarinal ( Fig. 7.6 ) or right paratracheal regions. Parenchymal bronchogenic cysts are typically sharply circumscribed, solitary, and round or oval and frequently located in the medial third of the lower lobes. Serial radiographs usually show little change in size and shape with time, although slow growth can sometimes be observed over years. Bronchogenic cysts typically do not communicate with the tracheobronchial tree until they become infected. When communication is established, the cyst may contain air, with or without fluid. Communication between a cyst and the tracheobronchial tree can produce a check-valve mechanism that may result in rapid expansion of the cyst.
Computed Tomography
In approximately 50% of cases the diagnosis of a benign cyst can be made confidently on CT, manifesting as a homogeneous fluid-density (0–20 Hounsfield units [HUs]) nonenhancing mass with a thin, smooth wall (see Fig. 7.6 ). Mediastinal cysts tend to conform to the adjacent mediastinal structures, whereas pulmonary cysts displace the adjacent parenchyma. Increased cyst attenuation may result from the presence of protein, hemorrhage, or calcium oxalate within the mucoid cyst contents and may make the cyst indistinguishable from other masses. When infected, the cysts may have inhomogeneous enhancement and resemble an abscess or necrotic lymph node. Occasionally, bronchogenic cysts are air filled and multilocular, mimicking an abscess. The lung adjacent to bronchogenic cysts is frequently abnormal and shows areas of decreased attenuation and scarring.
Magnetic Resonance Imaging
MRI is useful to determine the internal contents of cysts with equivocal CT features. Simple cysts typically manifest with low T1 and high T2 signal properties as cyst contents include predominantly serous fluid, whereas proteinaceous cysts may have higher T1 signal intensity. Regardless of the presence of high protein content, bronchogenic cysts characteristically have homogeneous high signal intensity (approximating that of cerebrospinal fluid) on T2-weighted sequences (see Fig. 7.6 ). When complicated by infection or hemorrhage, heterogeneous and variable signal intensity on both T1- and T2-weighted images may make the diagnosis less apparent.
Imaging Algorithm
Bronchogenic cysts are often first detected incidentally on routine chest radiography. However, it does not allow a confident diagnosis of bronchogenic cyst. Therefore CT or MRI is recommended for further evaluation. Because of the lack of radiation exposure and higher specificity, MRI is superior to CT in the evaluation of patients with suspected bronchogenic cysts. When neither CT nor MRI is diagnostic, the cystic nature can be confirmed by CT-guided needle aspiration of the cyst contents.
Differential Diagnosis
The differential diagnosis includes lung tumor, abscess, vascular malformation (e.g., arteriovenous malformation), and congenital pulmonary airway malformation. Bronchogenic cysts can usually be readily distinguished from lung tumor or vascular malformation by the characteristic water density on CT, homogeneous high signal intensity on T2-weighted MR images, and lack of enhancement. In the majority of cases these findings, together with the presence of a thin smooth wall, allow distinction of bronchogenic cysts from lung abscess. However, infected or hemorrhagic cysts may have heterogeneous appearance or signal intensity on CT and MRI, resembling lung abscesses. Bronchogenic cysts can generally be distinguished from congenital pulmonary airway malformations because they consist of a single cystic mass, whereas the latter usually consist of multiple cystic lesions with or without aberrant vascular supply.
Synopsis of Treatment Options
Despite the presence or absence of symptoms, management of bronchogenic cysts is typically surgical, in children and otherwise healthy young adults who are low risk for surgery. Although endobronchial cyst aspiration may be performed in symptomatic patients, surgical resection is usually necessary to prevent recurrence or rare malignant transformation. Nearly 50% of asymptomatic patients go on to develop symptoms or complications of the cyst, and malignancy has been found in 0.7% of cysts, justifying the aggressive surgical approach to these patients. However, conservative management may also be considered in asymptomatic adult patients in whom a bronchogenic cyst is incidentally detected and close clinical and radiographic follow-up is possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree