The current practice of breast imaging calls for using breast magnetic resonance imaging (MRI) only on high-risk patients with appropriate indications to maximize cancer detection yield and minimize the false-positive rate. Even with careful selection of patients, radiologists usually encounter benign findings more frequently than malignant lesions when interpreting breast MRI. It is important for radiologists to be familiar with these benign findings because it will enable them to select lesions for biopsy with improved specificity and to better assess radiologic-pathologic concordance of biopsy results. Some benign lesions, however, are simply indistinguishable from malignancy on imaging, and biopsy is necessary for histologic diagnosis. This chapter describes the MRI appearances of common and uncommon benign findings.
Benign Cystic Lesions
Duct Ectasia
Duct ectasia is a nonspecific dilation of the major subareolar ducts, with occasional involvement of smaller ducts. It has been found in women of all ages, and its etiology is unknown. Some consider all inflammatory processes as the initiating event, leading to destruction of the elastic network of the duct with subsequent duct ectasia and periductal fibrosis. Others suggest that duct dilation is the primary event, caused by obstruction of the ducts leading to secondary inflammation owing to leakage of duct contents.
Most patients are asymptomatic. Some may present with spontaneous and intermittent clear to yellow nipple discharge. Mammographically, the calcified proteinaceous secretions within dilated ducts appear as bilateral symmetric rodlike “secretory calcifications.” Ultrasonography will demonstrate dilated ducts filled with anechoic fluid, without or with debris.
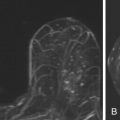
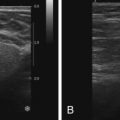
On MRI, ectatic ducts are usually incidental findings. They appear as non-enhancing, fluid-filled, often branching, tubular structures converging toward the nipple. The contents of the ducts may show signal intensities similar to that of water, hypointense on T1-weighted images and hyperintense on T2-weighted sequences. More often, the fluid is hyperintense in signal on T1-weighted images because of proteinaceous or hemorrhagic contents ( Figure 7.1 , A ). Because of such signal hyperintensity on precontrast images, postcontrast subtraction images are usually needed to confirm absence of contrast enhancement ( Figure 7.1 , B ). Focal enhancement within the duct lumen would raise concern for intraductal papilloma or carcinoma.

Fibrocystic Changes
Fibrocystic change (FCC) refers to a constellation of benign breast changes that represent normal but exaggerated, hormonally mediated breast tissue response. The histologic findings include cyst formation, apocrine metaplasia, mild epithelial hyperplasia, stromal fibrosis, and mild adenosis. It is common in younger women, affecting one third of females 20 to 45 years of age. There is no increased risk for the subsequent development of breast carcinoma in women with non-proliferative FCC. The risk of developing carcinoma is increased to 1.5 to 2 times when FCC is associated with moderate or florid ductal hyperplasia and increased 5 times with the presence of atypia.
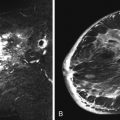
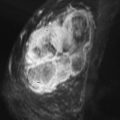
FCCs do not have specific mammographic or sonographic findings other than cyst formation. FCC has a wide spectrum of morphologic and kinetic features on MRI. It may appear as prominent nonmasslike enhancement in regional, focal, or segmental distribution ( Figures 7-2 and 7-3 ). A focal (nodular) form of FCC may appear as a mass with spiculated or smooth margins ( Figures 7-4 and 7-5 ). The degree of initial enhancement ranges from slow to rapid, and the kinetic curves may vary between washout, plateau, or persistent enhancement during the delayed phase. In a small series of 11 cases studied by Chen and colleagues, 62% of FCC showed early rapid enhancement with delayed washout, mimicking carcinoma.




Simple and Inflammatory Cysts
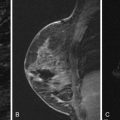
Cysts may be an isolated finding or may be seen as a component of FCCs on breast imaging. Simple cysts appear as circumscribed round or oval structures that are signal hyperintense on T2-weighted images and without contrast enhancement on postcontrast T1-weighted sequences ( Figure 7.6 ). Cysts associated with inflammation will show a thickened enhancing wall on postcontrast images ( Figure 7.7 ).


Benign Proliferative Lesions
Sclerosing Adenosis
Sclerosing adenosis is a benign proliferative lesion composed of distorted epithelial, myoepithelial, and stromal elements in a lobulocentric pattern. It may form a distinct nodule, hence the term nodular sclerosing adenosis or adenosis tumor .
Sclerosing adenosis is usually asymptomatic and is often an incidental microscopic finding in breast tissue removed for another indication. The nodular form may cause a palpable mass. Mammographically, sclerosing adenosis may present as clustered, round, or punctate microcalcifications; a focal asymmetry; architectural distortion; or a spiculated mass. The combination of these features may mimic carcinoma. Its sonographic appearance is also nonspecific, including masses with circumscribed, indistinct, or microlobulated margins.
On MRI, sclerosing adenosis may present as ductal enhancement or as homogeneously enhancing masses that are oval or round with lobulated or angular margins. According to Oztekin and coworkers , it shows intermediate signal intensity on T1- and T2- weighted images, with rapid initial enhancement and delayed persistent or wash-out kinetics. At our institution, both plateau and washout kinetics have been observed in biopsy-proved sclerosing adenosis. Figure 7.8 illustrates an example of nodular sclerosing adenosis showing as a mass.

Radial Sclerosing Lesion
Radial scar is a benign sclerosing breast lesion of unknown cause, characterized by a central fibroelastotic core with radiating ducts and lobules exhibiting various proliferative changes. The term complex sclerosing lesion is used in cases in which the lesion is larger than 1 cm or composed of several closely contiguous fibroelastotic areas. Rosen prefers the term radial sclerosing lesions for both entities. Radial sclerosing lesions are considered high-risk lesions because of their association with carcinomas. This entity will be covered in Chapter 8 .
Usual Ductal Hyperplasia
Ductal hyperplasia of usual type, or usual ductal hyperplasia (UDH), is a benign epithelial proliferation in a duct or lobule beyond the usual double cell layer, but no more than three to four cell layers above the basement membrane. It is a response to hormonal stimulation and imbalance. The term can be misleading, because this lesion usually occurs within the terminal ductal lobular unit (TDLU).
UDH is clinically silent and almost always detected on screening mammography as microcalcifications or a mass. It does not have specific sonographic presentation. No systematic review of the MRI appearance of UDH is available in the literature. Published images demonstrate either foci or nonmasslike enhancement (stippled or clumped) in a linear, ductal, or regional distribution ( Figure 7.9 ). Some biopsy-proved cases at our institution showed UDH as a sub-centimeter mass ( Figure 7.10 ). Delayed enhancement kinetics may be persistent, plateau, or mixed with some washout.


Columnar Cell Changes/Flat Epithelial Atypia
Columnar cell changes refer to a condition in which the normal epithelial cell layer of the TDLU is replaced by one or two layers of taller columnar epithelial cells that have basal nuclei and apical cytoplasmic snouts. When these columnar cells show cellular stratification with more than two cell layers, the entity is described as columnar cell hyperplasia. Columnar cell change with atypia and columnar cell hyperplasia with atypia are collectively referred to as flat epithelial atypia (FEA). Because of its frequent association with carcinomas, FEA is regarded as a high-risk lesion. This entity will be discussed in detail in Chapter 8 .
Atypical Ductal Hyperplasia
Atypical ductal hyperplasia (ADH) defines a group of proliferative lesions that have some, but not all, of the features of ductal carcinoma in situ (DCIS), either by lack of a major defining cytologic feature of carcinoma or by having the features to lesser extent than true DCIS. ADH is associated with a 4 to 5 times relative risk of development of breast cancer within 10 to 15 years. It will be covered in Chapter 8 .
Lobular Neoplasia
Atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) are relatively uncommon lesions, collectively identified as lobular neoplasia. These lesions are considered to be generalized risk factors for subsequent breast cancer, with a relative risk of 4 to 5 times for ALH and 8 to 10 times for LCIS. Lobular neoplasias are high-risk lesions that will be discussed in detail in Chapter 8 .
Fibroepithelial Tumors
Fibroadenoma
Fibroadenoma is a benign hyperplastic fibroepithelial tumor characterized by concurrent proliferation of both glandular and stromal elements in varying proportions. It is the most common breast mass encountered in women younger than 35 years of age and the most common solid mass in women of all ages. Juvenile (cellular) fibroadenoma is a variant of fibroadenoma that occasionally occurs in adolescents who present with a rapidly growing, well-defined mass that may reach 20 cm in size. The macroscopic and histologic appearances are similar to fibroadenomas, although the stroma may be more cellular. This makes differentiation from phyllodes tumor challenging in some cases.
A patient with a fibroadenoma usually presents with a mobile, painless, palpable mass. Mammographically, it appears as a circumscribed round, oval, or lobular mass that is iso- or hypodense to the parenchyma. Coarse calcifications, when present, may be diagnostic. Sonographically, it appears as a mass that is iso- or hypoechoic to fat, with a parallel orientation to the skin. Atypical findings include microlobulated or indistinct margins and acoustic shadowing due to internal calcification. In keeping with their histologic heterogeneity, fibroadenomas have been shown to have widely varied MRI appearances. On MRI, a fibroadenoma typically appears as an oval mass with smooth margins. It may have a lobular shape. On T1-weighted images, it is iso- or hypointense to normal parenchyma. On T2-weighted images, its signal intensity varies with the amount of myxoid (high signal) and fibrous (low signal) components. The fibrous component increases with increasing age. The degree of contrast enhancement varies with its degree of sclerosis and cellularity.
Nunes and coworkers described low-signal internal septations that are seen on T2- or enhanced T1- weighted images, representing dense collagen bands, as 89% to 93% specific for the diagnosis of fibroadenoma. The specificity increased slightly when internal septations were seen in combination with features of smooth margins or lobular shape. Figure 7.11 illustrates a fibroadenoma with these classic features. However, such septations are observed in only 40% to 64% of fibroadenomas on MRI. Furthermore, they have recently been described in benign and malignant phyllodes tumors and mucinous carcinoma and may be of limited value for diagnosing fibroadenoma when considered alone.

Fibroadenomas usually enhance homogeneously, with heterogeneous enhancement seen with increasing sclerosis. The most common enhancement kinetics of fibroadenoma is rapid (early phase) and persistent (delayed phase). However, some fibroadenomas are known to show rapid early enhancement with delayed washout kinetics, mimicking malignancy ( Figure 7.12 ).

When a fibroadenoma is suspected on MRI, review of the mammogram and sonogram may help in confirming the diagnosis. However, biopsy should be considered in cases in which a presumed fibroadenoma demonstrates interval growth or atypical features on conventional imaging.
Phyllodes Tumor
Phyllodes tumor is a rare neoplasm with a reported incidence of less than 1% of all female breast tumors. This tumor is similar to fibroadenoma in that it is a fibroepithelial lesion with both epithelial and stromal proliferations. However, phyllodes tumor has a much greater stromal cellularity than a fibroadenoma. Phyllodes tumors are high-risk lesions and will be covered in Chapter 8 .
Papillary Lesions
Papillary lesions of the breast may be classified as solitary intraductal papillomas, multiple intraductal (peripheral) papillomas, atypia-DCIS within a papilloma, micropapillary DCIS, and papillary carcinoma. Benign papillary lesions include solitary and multiple intraductal papillomas and papillomas with atypia. These are high-risk lesions and will be discussed in detail in Chapter 8 .
Mesenchymal Lesions
Pseudoangiomatous Stromal Hyperplasia
Pseudoangiomatous stromal hyperplasia (PASH) is an uncommon benign proliferation of fibrous stroma, containing slitlike pseudovascular spaces lined by myofibroblasts. PASH usually occurs in premenopausal women, probably resulting from an aberrant response to hormonal stimuli. Less commonly, it may occur in postmenopausal females on hormone replacement therapy or as an incidental component of gynecomastia in males. It is considered a neoplastic process because of its ability to recur. However, it is not known to be premalignant.
PASH is usually an incidental microscopic finding in breast biopsies with no mammographic correlate. When symptomatic, a patient with PASH may present with a painless, mobile mass (nodular PASH), frequently misdiagnosed as a fibroadenoma. It can become very large gradually or rapidly (tumoral PASH) or extensively infiltrative (diffuse PASH), causing dramatic asymmetric enlargement of the involved breast. As a mass, PASH may or may not have a discernible capsule. Rarely, skin necrosis can develop over a rapidly growing lesion.
Mammographically, nodular PASH may appear as a circumscribed or partially circumscribed mass, or as a developing focal asymmetry. Several authors have reported it as a mass with indistinct, obscured, or spiculated margins. No cases of architectural distortion or calcifications were identified.
Sonographically, PASHs usually appear as hypoechoic masses with rare cystic components, and they are often mistaken for fibroadenomas. A less common presentation is an echogenic area containing central linear hypoechoic areas or cysts. Multiple tiny cystic spaces within dense stroma have been reported in tumoral and diffuse forms of PASH. Abundant color flow on Doppler, indicating hyperemia in a diffuse PASH, has been observed. Teh and colleagues reported the presence of fine hyperechoic lines in a “lacelike” pattern, corresponding with the dense collagen strands in tumoral PASH. The posterior acoustic features vary from moderate acoustic enhancement to mild shadowing.
As expected with its various forms, PASH has a variable MRI appearance. In the series of Jones and associates, most lesions present as clumped nonmasslike enhancement, in focal or segmental distributions ( Figure 7.13 ). Nodular PASH may appear as a small irregular mass ( Figure 7.14 ).


Only sporadic single case reports are available on the MRI appearance of tumoral or diffuse PASH. Tumoral PASH appears as a large circumscribed mass or a mass without a discernible capsule. On T1-weighted sequences, the mass shows intermediate signals with interspersed cysts of lower signals. On T2-weighted sequences, high-signal cysts mixed with low- to intermediate-signal stroma are reported. According to Teh and coworkers, hyperintense fine reticular “lacelike” networks on T2-weighted images, thought to correspond to collagen strands, may be a distinguishing MRI feature for tumoral PASH. Enhancement appears heterogeneous on published images, with delayed persistent kinetics ( Figure 7.15 ).

Two cases of bilateral diffuse PASH with similar MRI characteristics were found in the English literature. Baskin and associates reported T2 prolongation of prominent stroma between glands on fat-suppressed T2-weighted spin echo images and diffuse prominent enhancement of individual glands throughout the breast. Time-intensity curves show initial rapid and delayed persistent enhancement kinetics.
Most reports describe delayed persistent enhancement kinetics in PASH, especially in the tumoral or diffuse form. However, in cases of PASH presenting as small irregular masses, plateau or washout delayed kinetics have also been observed (see Figure 7.14 ).
PASH is not premalignant, but may recur locally after excision in 12.5% of cases. Although it was not known to be associated with malignancy previously, Drinka and colleagues recently identified proliferative PASH in 2.4% of 79 PASH cases they studied, with atypical ductal and atypical lobular hyperplasia found in 25.6% and infiltrating carcinoma in 11% of proliferative PASH. Hence, they recommend thorough histologic evaluation of biopsy specimen demonstrating PASH, with clinical and radiologic follow-up. The recommended treatment is wide local excision to prevent local recurrence. Rarely, diffuse PASH may necessitate mastectomy for symptomatic and cosmetic indications.
Stromal Fibrosis
Stromal fibrosis is a benign lesion characterized by proliferation of fibrous stroma with obliteration and atrophy of mammary ducts and lobules. It has been described by various terms such as focal fibrous disease , fibrous tumor , focal fibrosis , and fibrous mastopathy . The diagnosis has become more frequently encountered with increased use of screening mammography, sonography, and breast MRI. Stromal fibrosis may represent 2.1% to 9% of lesions found in image-guided core biopsy.
Clinically, a palpable mass may be found in a patient or the lesion may be seen as a screening mammographic abnormality in an asymptomatic woman. Its mammographic findings are nonspecific, including spiculated mass, architectural distortion, or a benign-appearing circumscribed mass. Sonographically, it may be highly echogenic, similar to normal fibroglandular tissue, or it may present as a circumscribed or spiculated hypoechoic mass. Biopsy is often needed for diagnosis.
Lee and Mahoney studied 40 cases of biopsy-proved, pure stromal fibrosis on breast MRI. More than half of the lesions presented as masses ( Figure 7.16 ), 87% of which were less than 1 cm in size ( Figure 7.17 ). Some appeared as foci or areas of nonmasslike enhancement ( Figure 7.18 ). Although extremely variable, the most common appearance of nonmass lesions was clumped enhancement in linear distribution.



Most lesions were iso- to hypointense to normal parenchyma on T1-weighted images, and variable in signal intensity on T2-weighted sequences, depending on the water content of the lesion. The enhancement kinetics also varied but usually showed medium or rapid initial contrast uptake, with plateau or washout delayed curves. On histopathology, ectatic vessels were found within most of the lesions, which may explain the degree of contrast enhancement on MRI.
Mammary Fibromatosis
Mammary fibromatosis is a rare benign stromal tumor of the breast that accounts for less than 0.2% of all breast tumors. It was initially reported in a patient with Gardner syndrome but may occur sporadically or after trauma or surgery. Most cases were reported in women, with rare cases in men.
Mammary fibromatosis mimics malignancy, both clinically and by imaging. Clinically, it presents as a suspicious palpable mass, which may be associated with skin retraction and fixation to the chest wall. It appears as a spiculated mass on mammogram. On ultrasound, it usually appears as an irregular solid hypoechoic mass with spiculated or microlobulated margins and tethering of Cooper ligaments. It may be locally aggressive, involving the pectoralis or intercostal muscles.
On MRI, mammary fibromatosis typically appears as an irregular mass isointense to muscle on T1-weighted images and variably hyperintense on T2-weighted sequences. It typically shows benign persistent enhancement curves in the delayed phase ( Figure 7.19 ), although plateau and washout kinetics have been reported. MRI is the best imaging modality for evaluation of chest wall invasion and extent of disease. The recommended treatment is complete surgical resection with clear margins. Although benign, it is locally aggressive with a high recurrence rate of up to 29%.

Granular Cell Tumor
Granular cell tumor (GCT) is a tumor of Schwann cell origin that may occur at any body site, involving the breast in 6% to 8% of cases. It is an uncommon but important entity because of its tendency to mimic breast carcinoma on clinical and imaging presentations.
Clinically, a patient with GCT usually presents with a palpable firm lump, occasionally associated with skin fixation and thickening and nipple retraction. It has a widely varied mammographic appearance, ranging from a focal asymmetry to a mass with ill-defined or spiculated margins. Its sonographic features also vary widely from benign-appearing masses with circumscribed margins and acoustic enhancement to malignant-appearing masses with spiculated margins and acoustic shadowing. Histologically GCTs with circumscribed margins exhibit scant fibrous stroma, whereas the ones with spiculated margins have a background of dense fibrous stroma.
There are few reports of the MRI appearance of GCT. It has been described as a mass with either smooth or spiculated margins. It is low in signal intensity on T1-weighted images, hypointense to glandular tissue or isointense to skeletal muscle. The reported T2 signal intensities and enhancement patterns of GCT are widely variable. On T2-weighted sequences, its signal intensity is described as hypointense to muscle, isointense to glandular tissue, or demonstrating a high–signal-intensity rim. It may enhance homogenously or heterogeneously, particularly pronounced at its margin. GCTs show medium or rapid rate of contrast enhancement during early phase, with either persistent or washout kinetics in the late phase. Figures 7-20 and 7-21 are examples of GCTs identified on MRI.


It is impossible to establish a diagnosis of GCT without biopsy. Despite its diagnostic challenges, GCT is usually benign and carries an excellent prognosis. However, approximately 2% of GCTs are malignant. Malignant GCT can metastasize widely and have a grave prognosis. Wide local excision is the treatment of choice for both benign and malignant GCTs. Long-term surveillance is recommended for large tumors, rapid growth, local recurrence, and multiple lesions, owing to the slight, but real, malignant potential of GCTs.
Diabetic Mastopathy
Diabetic mastopathy is a condition in which stromal proliferation forms fibrous masses, predominantly in female patients with long-standing insulin-dependent (type 1) diabetes mellitus. Rarely, it may occur in males or in type 2 diabetic patients. The interval between the onset of diabetes and presentation of the breast lesion is reportedly about 20 years. It is known by various names, including diabetic fibrous mastopathy , mastopathy in insulin-dependent diabetes, diabetic fibrous breast disease , and diabetic sclerosing lymphocytic lobulitis of the breast .
The pathogenesis of diabetic mastopathy is not completely understood. It probably represents an immune reaction to the abnormal accumulation of altered extracellular matrix in the breast, secondary to prolonged hyperglycemia. The characteristic pathology findings include dense keloid-like fibrosis enclosing “epithelioid fibroblasts,” lymphocytic ductitis and lobulitis, and perilobular and perivascular lymphocytic infiltration. The lymphocytic infiltrate is composed predominantly of B cells. The increased expression of HLA-DR4 antigen in involved lobular epithelium and the association with other autoimmune diseases support the presumed autoimmune pathogenesis.
Clinically, patients usually present with a nontender, palpable, firm to hard mass in one or both breasts. Mammography may reveal a heterogeneously dense parenchymal pattern, focal asymmetry, or a mass with obscured, indistinct, or spiculated margins. Ultrasound shows a heterogeneously hypoechoic mass with ill-defined margins and marked posterior acoustic shadowing. There is absence of color flow signals on Doppler study. Diabetic mastopathy may mimic breast cancer on clinical, mammographic, and ultrasound examinations because of the extensive fibrosis. Tissue sampling is necessary for diagnosis and to exclude a malignancy. Treatment options are either excision or close follow-up, depending on the individual circumstances.
Diabetic mastopathy is isointense to glandular tissue on breast MRI, but a mass can often be appreciated on the non–fat-suppressed T1-weighted image. Wong and colleagues and Tuncbilek and coworkers reported no discernible enhancing mass on postcontrast MRI. Wong and colleagues described nonspecific patchy or diffuse stromal enhancement, and Tuncbilek and associates reported homogeneous, low enhancement and glandular distortion. Figure 7.22 illustrates a published case of diabetic mastopathy.

Lipoma
Lipoma is a benign fatty tumor that may occur anywhere in the breast. Most lipomas are located in the subcutaneous fat. They may manifest as soft, mobile palpable masses or incidental findings on screening mammography. The cause of lipomas is unknown.
Lipomas have a classic appearance on mammography, presenting as a completely radiolucent mass with a thin radiopaque capsule. On ultrasound, they may be isoechoic to adjacent fat lobules or mildly hyperechoic. Occasionally, lipomas may contain multiple echogenic septa that course parallel to the skin.
On MRI, lipomas present as oval masses with smooth margins. Their signal intensity follows that of normal fatty tissue in the breast on all sequences. They have high signal intensity identical to the adjacent normal fatty tissue on T1-weighted non–fat-suppressed sequences. On T1-weighted fat-suppressed sequences, their signals are suppressed to the same degree as the adjacent normal fat ( Figure 7.23 ).

Hamartoma
Hamartomas are benign breast lesions composed of a variety of normal breast constituents, including fat, glandular tissue, and fibrous connective tissue. Other names, such as fibroadenolipoma or lipofibroadenoma have been used to reflect the dominant tissue types within the mass. The lesion may be a result of dysgenesis rather than a true tumor. Most of hamartomas are detected in pre- and perimenopausal women. They are variable in size but usually measure several centimeters in diameter. Clinically, hamartomas are found either incidentally on screening mammograms or in patients presenting with painless palpable masses.
On mammograms, hamartomas may have a classic appearance of a circumscribed mass containing both fat and soft tissue density surrounded by a thin radiopaque capsule. When a hamartoma contains a very small amount of fat, it may mimic a fibroadenoma or circumscribed carcinoma. Its sonographic appearance is also variable. It often appears as a mass with smooth margins, containing areas of low-level internal echogenicity interspersed with irregular areas of hyperechogenicity.
On MRI, a hamartoma appears as an encapsulated mass with a heterogeneous appearance. Some contrast enhancement can be seen in the glandular elements on T1-weighted postcontrast images ( Figure 7.24 ).