(1)
Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
(2)
Neuroscience Program, Harvard Medical School, Boston, MA, USA
Abstract
In vivo bioluminescence imaging (BLI) is a powerful technology that gives information on biological processes in living animals over multiple time points. Importantly BLI can also yield anatomical localization of signal which can provide important information when performing biodistribution studies of different macromolecules. This is of particular interest for gene therapy vectors such as adeno-associated virus (AAV) vectors in which knowledge of in vivo gene expression profiles help characterize what target tissues or organs the vector may be useful for. It can also be utilized to assess novel vector systems for their ability to overcome specific in vivo barriers of effective gene therapy. Here we describe BLI of AAV-encoded firefly luciferase (Fluc) expression in mice after intravascular delivery. This protocol can be amended for use with different virus vectors (e.g., lentivirus, adenovirus) as well as nonviral gene delivery (e.g., plasmid DNA, liposomes).
Key words
In vivo bioluminescence imagingFirefly luciferaseVirus vectorAdeno-associated virus (AAV)Optical imagingMouse models1 Introduction
In vivo bioluminescence imaging (BLI) has provided in depth knowledge of biological processes such as metastasis [1], transcription factor activation [2], and protein–protein interactions [3]. BLI has become an important tool of gene therapy as it allows a real-time kinetic analysis of gene expression in individual animals [4]. Firefly luciferase (Fluc) is one of the most widely used luciferases for BLI for several reasons. First, the half-life of Fluc is short [5] (2–3 h) allowing accurate measurement of ongoing processes without accumulation effects observed with fluorescent reporters with longer half-lives [6]. Second, there is a relatively inexpensive, water-soluble version of its substrate, d-luciferin, that permits relatively large amounts of substrate to be injected intraperitoneally (i.p.), which is a technically simpler injection route than intravenous (i.v.) injection. Finally since Fluc is a cytoplasmic protein, the enzyme and catalyzed light emission are localized to the expression site.
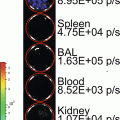
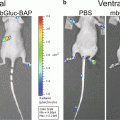
Virus vectors based on adeno-associated virus (AAV) mediate high levels of transgene expression in several preclinical models, including nonhuman primates [7, 8]. In the field of neuroscience, AAV is the most powerful vector tool due to its robust gene transfer to cells of the CNS [9]. Recently several serotypes of AAV have been shown to cross the blood–brain barrier in mice and non-human primates, leading to broad-spread gene delivery throughout the brain [10, 11]. Despite the promising results with AAV there are several in vivo biological barriers which hinder the vector for certain applications in the human population. For example, neutralizing antibodies (approximately 30 % of people in North America have neutralizing antibodies to AAV2 [12]) can abrogate gene transfer after i.v. injection. Using BLI to assess novel AAV serotypes or genetically engineered vectors ability to transduce liver in the presence of neutralizing antibodies is a rapid approach to screening multiple vectors. Combined with histological analysis, BLI is an excellent component in the evaluation of the in vivo transduction profiles of virus vectors including AAV.
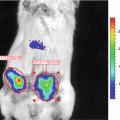
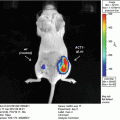
Here we describe a method for evaluating distribution of transgene expression of AAV-encoded Fluc in mice after tail vein injection.
2 Materials
2.1 Virus Components
1.
293T cells (passage 1–30).
2.
Dulbecco’s Modified Eagles Medium (DMEM) (high glucose) supplemented with 10 % fetal bovine serum and 1 % Penicillin/Streptomycin (p/s).
3.
Dulbecco’s Modified Eagle Medium (high glucose) supplemented with 2 % Fetal bovine serum and 1 % Penicillin Streptomycin.
4.
15 cm diameter tissue culture dishes.
5.
Trypsin.
6.
Phosphate buffered saline.
7.
2 M CaCl2.
8.
2.5 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer.
9.
2× HEBS (HEPES buffered saline) pH 7.0–7.05
Final concentration: 280 mM NaCl, 50 mM HEPES, 1.5 mM Na2HPO4.
10.
Freeze–Thaw Buffer (150 mM NaCl, 50 mM Tris–HCl pH 8.4).
11.
Plasmids for AAV production: (A) AAV plasmid containing transgene cassette (promoter and enhancers, gene of interest, poly A tail) flanked by viral inverted terminal repeats (ITRs), in this study we use AAV encoding Fluc; (B) AAV rep/cap plasmid encoding the replication proteins of AAV and the serotype capsid (in this protocol we use AAV serotype 9); (C) a helper plasmid encoding Ad helper genes necessary for AAV replication (see Notes 1 and 2).
12.
CO2 incubator for 293T tissue culture and vector production.
13.
Tabletop centrifuge.
2.2 Iodixanol Gradient Purification
1.
Dry ice and ethanol.
2.
Benzonase.
3.
Iodixanol for density gradient centrifugation (known as OptiPrep).
4.
Type 70Ti rotor ultracentrifuge tubes (39 ml max vol).
5.
16 gauge needles.
6.
10 ml syringes.
7.
Beckman Coulter Optima™ L-90K Ultracentrifuge with 70Ti fixed angle rotor.
8. Preparing iodixanol gradient solutions:
0.5 % Phenol Red: 0.25 g in 50 mL 50 % EtOH.
15 % Iodixanol: 12.5 mL 60 % Solution + 5 mL 10× PBS + 10 mL 5 M NaCl + 50 μl
1 M MgCl + 125 μl 1 M KCl + 75 μl 0.5 % phenol red + H2O up to 50 mL.
25 % Iodixanol: 20.83 mL 60 % iodixanol solution + 5 mL 10× PBS + 50 μl.
1 M MgCl2 + 125 μl 1 M KCl + 100 μl 0.5 % phenol red + H2O up to 50 mL.
40 % Iodixanol: 33.4 mL 60 % iodixanol solution + 5 mL 10× PBS + 50 μl.
1 M MgCl2 + 125 μl 1 M KCl + H2O up to 50 mL.
60 % Iodixanol: 25 mL 60 % iodixanol solution + 25 μl.
1 M MgCl2 + 65.5 μl 1 M KCl + 12.5 μl 0.5 % phenol red.
2.3 Virus Concentration
1.
Buffer B (20 mM Tris–HCl, 500 mM NaCl, pH 8.5).
2.
Amicon Ultra 100 kDa MWCO filters (Millipore, UFC910096).
3.
Pierce Slide-A-Lyzer MINI Dialysis Device, 20 kDa MWCO, 2 mL.
4.
0.22 μm Millex-GV Filter Unit filters (Millipore, slgv004sl, hold up volume <10 μl).
2.4 Quantitative PCR (QPCR)
1.
Nuclease-free water.
2.
AAV primers and Taqman probe which bind to the PolyA tail region in transgene cassette.
Primers sequence:
F2: 5′CCTCGACTGTGCCTTCTAG3′
R2: 5′TGCGATGCAATTTCCTCAT3′
Probe sequence: 5′-6FAM-TGCCAGCCATCTGTTGTTTGCC—MGB (minor groove binding)
3.
QPCR master mix.
4.
96 well plate.
5.
Plate covers.
6.
Applied Biosystems 7500 quantitative PCR thermal cycler.
2.5 Intravenous Injection Components
1.
Mouse restrainer.
2.
40 °C water in a beaker.
3.
28 gauge ½ in. insulin syringes.
4.
70 % alcohol wipes.
5.
Sterile gauze.
2.6 Luciferase Substrate
d-luciferin sodium or potassium salt. Suspend lyophilized powder to 25 mg/ml in sterile PBS pH 7.2–7.4. Store 0.5–1.5 ml aliquots at −80 °C protected from light.
2.7 Anesthesia and Imaging System
1.
Perkin Elmer IVIS® Spectrum optical imaging system.
2.
XGI-8 gas anesthesia system.
3.
Living Image® software.
3 Methods
3.1 Production, Purification, and Quantitation of AAV Vector
Day 1:
Plate fifteen 15 cm plates with 1 × 107 293T cells per plate in DMEM 10 % FBS, 1 % p/s (20 ml of media/plate).
Day 2: See Note 3.
1–2 h before transfection, replace media with 20 ml of fresh DMEM 10 % FBS, 1 % Pen/Strep.
Prepare transfection mix (below is for 1 plate).
Tube A
Amount/volume (μg)
Tube B
Volume
AAV plasmid w/gene of interest
9.94
780 μl
Ad helper plasmid
25.56
rep/cap plasmid
12.37
2 M CaCl
96.9 μl
2.5 mM HEPES
Up to 780 μl
Total volume
780 μl
Total volume
780 μl
Add Tube A to Tube B drop-wise while vortexing Tube B. Vortex for 1 min.
Incubate mix at room temperature for 20 min. A slightly cloudy mixture should be visible.
Add ~1.5 ml of virus mix per 15 cm plate, distributing drop-wise over the surface of the plate. Tilt plate side to side, front to back, to mix evenly and incubate overnight.
Day 3:
Replace media with DMEM 2 % FBS 1 % Pen/Strep.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree