Tobacco use
Exposure to aromatic amines and aniline dyes
Arsenic ingestion
Cyclophosphamide treatment
Recurrent severe urinary tract infections, indwelling catheters, or stones (squamous cell carcinoma)
Schistosomiasis (squamous cell carcinoma)
Urachal remnant and cystitis glandularis/bladder extrophy (adenocarcinoma)
Pelvic Radiation
Diagnosis
Anatomy
The urinary bladder wall consists of four layers: (1) urothelium lining the bladder lumen; (2) the vascular lamina propria; (3) the muscularis propria consisting of bundles of smooth detrusor muscle; and (4) the outermost adventitia formed by the connective tissue. The bladder is surrounded by pelvic fat and its dome is covered by the peritoneum.
More than 95 % of bladder tumors arise from the urothelium. They include urothelial carcinomas (>90 %), squamous cell carcinomas (5 %), and adenocarcinomas (<2 %). Other rare epithelial tumors are metastases, small cell or neuroendocrine carcinoma, carcinoid tumors, and melanoma.
Less than 5 % of bladder tumors are mesenchymal in origin. Benign mesenchymal bladder tumors include leiomyoma (most common), paraganglioma, fibroma, plasmocytoma, hemangioma, solitary fibrous tumor, neurofibroma, and lipoma. Malignant mesenchymal bladder tumors are rhabdomyosarcoma, leiomyosarcoma, lymphoma, and osteosarcoma.
Presentation
Bladder cancer may be detected incidentally or because of symptoms. Found in about 85 % of patients, intermittent gross painless hematuria is the most common presenting symptom. Unexplained irritative voiding symptoms such as increased urinary frequency, urgency, or dysuria may also be a sign of bladder cancer, particularly carcinoma in situ (Tis). Flank pain and other symptoms related to hydronephrosis can be present with tumors involving the ureterovesical junction.
Staging
Clinical Staging
Clinical staging of bladder cancer is an important but imperfect evaluation with a 30–50 % rate of understaging at the time of cystectomy [7]. When bladder cancer is suspected, the initial work-up consists of voided urine cytology, cystoscopy, and radiological evaluation of the upper tracts with computed tomography urography (CTU) or magnetic resonance urography (MRU). Voided urine cytology is the noninvasive diagnostic test of choice but suffers from low sensitivity, particularly for low-grade tumors [8]. Conventional or white-light cystoscopy—with endoscopic evaluation of the bladder and urethra—is the “gold standard” for the detection of bladder cancer. Tissue from any suspicious area is removed with either bladder biopsy or transurethral resection of bladder tumor (TURBT).
TURBT should include muscularis propria, especially if the tumor is high grade or lamina propria is involved. If TURBT demonstrates lamina propria invasion but the specimen does not have sufficient muscularis propria, a repeat resection is required to exclude muscle-invasive disease. A second TURBT should be considered in all patients with high-grade or lamina propria invasive tumors to prevent understaging and possible progression to metastatic disease [9].
During TURBT, a bimanual examination is done to assess the degree of mobility of the bladder and other pelvic organs. In the setting of muscle-invasive disease, a metastatic work-up should be performed including chest, abdomen, and pelvic CT, liver function tests, and assessment of serum creatine and electrolytes.
Pathologic Staging
Histologic staging of bladder cancer is determined by the tumor-node-metastasis (TNM) staging system (see Tables 3.2 and 3.3). Tumor (T) stage is based on the extent of primary tumor penetration into the bladder wall [10].
Table 3.2
TNM staging of bladder cancer
Primary Tumor (T) |
Tx Primary tumor cannot be assessed |
T0 No evidence of primary tumor |
Ta Noninvasive papillary carcinoma |
Tis Carcinoma in situ: “flat tumor” |
T1 Tumor invades subepithelial connective tissue |
T2 Tumor invades muscularis propria |
pT2a Tumor invades superficial muscularis propria (inner half) |
pT2b Tumor invades deep muscularis propria (outer half) T3 Tumor invades perivesicular fat |
pT3a Microscopically |
pT3b Macroscopically (extravesical mass) |
T4 Tumor invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, or abdominal wall |
T4a Tumor invades prostatic stroma, uterus, or vagina |
T4b Tumor invades pelvic wall or abdominal wall |
Regional Lymph Nodes (N) |
Nx Lymph nodes cannot be accessed |
N0 No lymph node metastasis |
N1 Single regional lymph node metastasis in the true pelvis (internal iliac, obturator, external iliac, or presacral lymph node metastasis) |
N2 Multiple regional lymph node metastasis in the true pelvis N3 Lymph node metastasis to the common iliac lymph nodes |
Distant Metastasis (M) |
M0 No distant metastasis |
M1 Distant metastasis |
Table 3.3
Anatomic stage/prognostic groups
Stage 0a |
Ta, N0, M0 |
Stage 0is |
Tis, N0, M0 |
Stage I |
T1, N0, M0 |
Stage II |
T2a, N0, M0 |
T2b, N0, M0 |
Stage III |
T3a, N0, M0 |
T3b, N0, M0 |
T4a, N0, M0 |
Stage IV |
T4b, N0, M0 |
Any T, N1-3, M0 |
Any T, Any N, M1 |
Urothelial carcinomas comprise the vast majority of bladder tumors (90 %) and are classified into four categories by the World Health Organization (WHO) and the International Society of Urologic Pathology consensus: (1) papilloma; (2) papillary urothelial neoplasm of low malignant potential; (3) low-grade carcinoma; and (4) high-grade carcinoma [11].
Of all newly diagnosed urothelial carcinomas, 70 % present as non–muscle-invasive (superficial) tumors (stages Ta, Tis, or T1) and 30 % as muscularis propria invasive disease (stages T2–T4), which is associated with a high risk of distant metastases. Around 50–70 % of non-muscle-invasive tumors recur and 10–20 % progress to muscularis propria invasive disease [7]. It remains a challenge to predict which patients will progress from non–muscle-invasive to muscle-invasive disease.
The TNM staging system provides important prognostic information that helps to predict outcome and guide clinical management. Several additional predictive factors (not included in the TNM staging system) such as tumor grade, multifocality, recurrence at 3 months, presence of Tis, and size of the initial tumor (>3 cm) should also be considered in the clinical decision making [12, 13].
Management
Non–muscle-Invasive Bladder Cancer
Current data suggest that patients with all types of non–muscle-invasive bladder cancer benefit from one-time intravesical instillation of a chemotherapeutic agent immediately after TURBT because of the decrease in the rate of recurrence [14].
Patients with high-risk disease (high-grade Ta, multifocal Tis, T1 tumors with associated Tis, and tumors that rapidly recur after TURBT) are candidates for further adjuvant intravesical drug therapy. Compared to other chemotherapeutic agents, intravesical Bacillus Calmette-Guerin (BCG) therapy is the most effective in the prevention of recurrence, but its role in slowing down disease progression is controversial [15, 16].
Close clinical follow-up is required for patients with non–muscle-invasive bladder cancer because of a high rate of recurrence and a risk of disease progression. After initial TURBT, patients should have follow-up cystoscopy and voided urine cytology every 3 months for 2 years, then every 6 months for 2 years, and then annually indefinitely. Upper tract imaging should be done every 12–24 months because of the 5 % lifetime risk for development of upper tract urothelial carcinoma after a diagnosis of bladder cancer [17].
Muscle-Invasive Bladder Cancer
The standard treatment for muscularis propria invasive bladder cancer is radical cystoprostatectomy in men and anterior exenteration (including the bladder, urethra, uterus, and anterior vaginal wall) in women. Additionally, in men, urethrectomy is performed if the prostatic stroma is invaded or Tis is present. In women, the uterus, vagina, and urethra are occasionally spared if they are uninvolved by the tumor and an orthotopic continent urinary diversion is planned.
Pelvic lymphadenectomy is done in all cases of radical cystectomy. Many investigators also advocate an extended lymphadenectomy that includes aortic bifurcation cranially and presacral lymph nodes caudally because it improves tumor staging and survival [18, 19].
After radical cystectomy, a segment of bowel is used for either noncontinent (ileal conduit) or continent (orthotopic neobladder or an abdominal pouch) urinary diversion. The ileal conduit is usually done in elderly patients with multiple comorbidities and increased operative risk; the continent diversion is preferred in young and healthy patients. At present, the role of neoadjuvant and adjuvant chemotherapy for improving survival is still uncertain [20–23].
Alternative treatment strategies for well-selected patients with muscle-invasive disease who strongly desire bladder preservation include TURBT alone, combined chemotherapy and radiation therapy, or trimodality approach consisting of TURBT, chemotherapy, and radiation. Further studies are needed to determine whether bladder preservation can occur while obtaining survival rates similar to those achieved after radical cystectomy.
Patients with muscle-invasive bladder cancer must be closely followed because up to 50 % of patients undergoing radial cystectomy for urothelial carcinoma will develop local or metastatic recurrence, typically within the first 2–3 years after surgery [24]. According to the National Comprehensive Cancer Network (NCCN) bladder cancer guidelines, after cystectomy patients should have urine cytology, electrolytes, and creatine every 3–6 months as well as upper tract and chest, abdomen, and pelvis imaging every 3–12 months for 2 years based on the risk of recurrence and then as clinically indicated [25].
Imaging Techniques
Table 3.4
Role of imaging
Evaluation of patient with hematuria and detection of urothelial lesions |
Intravenous Urography (IVU) has largely been replaced by CT Urography (CTU) |
MR Urography is preferred in patients with renal insufficiency |
Exception: patients with severely impaired renal function due to concern for nephrogenic systemic fibrosis (NSF) |
Pretreatment staging of muscle-invasive bladder cancer |
Imaging is indicated only if it will affect clinical management |
Purpose of imaging |
Assess the extent of local tumor invasion |
MRI is superior to contrast-enhanced CT (CE-CT) |
Better soft tissue resolution leading to higher accuracy in assessing depth of bladder wall and adjacent organ invasion |
Multiplanar capabilities resulting in improved visualization of bladder base and dome tumors |
Detection of nodal and distant metastases At present, CE-CT is the preferred imaging modality |
In the future, MRI with ultrasmall superparamagnetic iron oxide (USPIO) contrast agents may improve detection of metastatic lymph nodes |
CTU or MRU serves as a one-stop-shop examination |
Evaluates upper urinary tract to exclude synchronous urothelial lesions and assesses abdomen and pelvis for nodes and distant metastases |
Bone scintigraphy or focused MRI |
If bone pain or abnormal biochemical markers (serum calcium and alkaline phosphatase) |
Role of 18 F-FDG-PET |
Refer to Chap. 9 |
Post-treatment follow-up of patients with non-invasive and muscle-invasive bladder cancer |
Detection of post-surgical or post-treatment complications |
Evaluation for tumor recurrence or tumor progression |
Exclusion of metachronous upper tract and bladder urothelial carcinoma |
Table 3.5
CT: imaging techniques
Routine CE-CT is useful for detecting nodal or distant metastases but may be inadequate for detection and staging of urothelial lesions |
CTU is a one-stop-shop evaluation |
Examines entire urinary tract and diagnoses possible causes of hematuria |
Detects and stages upper tract and bladder urothelial lesions |
Evaluates the rest of the abdomen and pelvis |
Refer to the imaging technique section in chapter 2 for more information on CTU techniques |
Virtual cystoscopy |
Obtained by manipulating CTU data acquired during the excretory phase through the contrast-filled bladder |
Allows navigation within a three-dimensional model Further investigations are needed to study its added value |
Table 3.6
MRI: imaging techniques
Patients should void 1–2 h prior to the scan |
Prevents under or overdistention of the urinary bladder which compromise image quality |
Phase-array pelvic coil |
Improves signal-to-noise ratio |
Enables to obtain smaller field-of-view, high resolution images |
T1-WI |
Helps to demonstrate bladder cancer extension into perivesicular fat and to evaluate for pelvic adenopathy and osseous metastases |
T2-WI in three orthogonal planes |
Optimizes tumor detection and staging |
Plane(s) orthogonal to the tumor-bladder interface most accurately demonstrates the presence and extent of the bladder wall invasion |
DWI (b values: 0 and 1,000 s/mm2) |
Potential to improve tumor detection and staging accuracy |
Multiphasic contrast-enhanced MRI |
Improves tumor detecting and local staging such as assessment of depth of bladder wall invasion and adjacent organ involvement |
Normal Anatomy
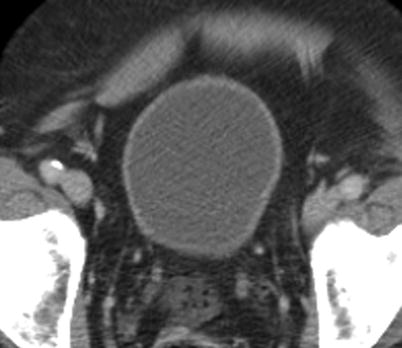
Fig. 3.1
CT appearance of normal urinary bladder. Four layers of the urinary bladder wall (mucosa, submucosa, detrusor muscle, and serosa) are not resolved on CT. As demonstrated on this axial CE-CT image, normal urinary bladder should have smooth wall with thickness varying with distention. Perivesicular fat should be pristine
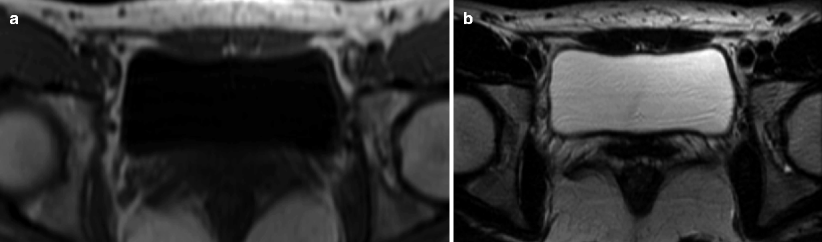
Fig. 3.2
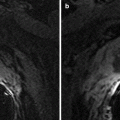
MR appearance of normal bladder. Axial T1-weighted image (a) and axial T2-weighted image (b). Like other stagnant fluids, urine has low signal intensity (SI) on T1-WI and high SI on T2-WI sequences. Urinary bladder wall has homogenous low-to-intermediate T1 SI and low T2 SI due to dominant detrusor muscle component. Mucosa, submucosa, and serosa are not normally seen as distinct layers. Submucosal edema is hyperintense to detrusor muscle and mildly hypointense to urine on T2-WI. In the presence of submucosal edema, mucosa is seen as thin low SI layer surrounding the bladder lumen. Bladder mucosa, submucosa, and urothelial carcinoma enhance on early dynamic postcontrast images, whereas the detrusor muscle enhances on delayed images
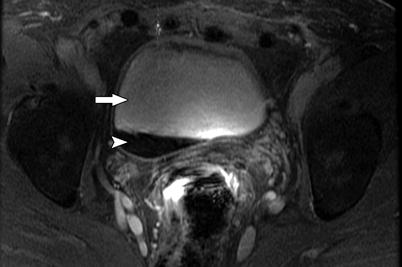
Fig. 3.3
MR illustration of T2-shortening and susceptibility effect of concentrated gadolinium. Gadolinium is a paramagnetic substance and produces both T1 and T2 shortening. As demonstrated on this contrast-enhanced T1-WI image, dilute gadolinium is hyperintense on T1-WI (arrow), whereas concentrated gadolinium may have lower SI on T1-WI (arrowhead) due to T2-shortening and susceptibility effect
Diagnosis
Cystoscopy is the “reference standard” |
Detection of bladder cancer with multidetector CTU |
Sensitivity 79–93 % |
Specificity 91–99 % |
High specificity (96 %) and negative predictive value (98 %) of CTU in patients with hematuria and no history of bladder cancer Normal urinary bladder evaluation on CTU may eliminate the need for further evaluation with cystoscopy |
Patients with CTU findings suspicious for bladder cancer may go directly to rigid cystoscopy, biopsy, and/or resection omitting preliminary evaluation with flexible cystoscopy |
Accuracy of CTU for bladder cancer detection is substantially lower in patients with a prior bladder cancer (78 %) because of post treatment changes from TURBT or intravesical therapy |
In this patient population cystoscopy is the test of choice |
Factors limiting detection of bladder cancer on CTU |
Bladder underdistention |
Pseudo-thickening of the bladder wall |
Bladder overdistention |
Flattening of some tumors |
Tumor characteristics Size <1 cm |
Carcinoma in situ which is typically plaque-like in appearance |
Tumor location |
Bladder neck, especially if benign prostatic hypertrophy |
DW- MRI is a new useful technique for bladder cancer detection in patients with hematuria and no history of bladder cancer |
Tumor appears as high signal intensity mass on high b-value DW-MR images |
Sensitivity, 98.1 %; specificity, 92.3 %; PPV, 100 %; NPV, 92.3 %; accuracy, 97 % [28] |
Excellent correlation between DW-MRI and cystoscopic findings (k = 0.94) [28] |
Imaging Appearance of Urothelial Carcinomas
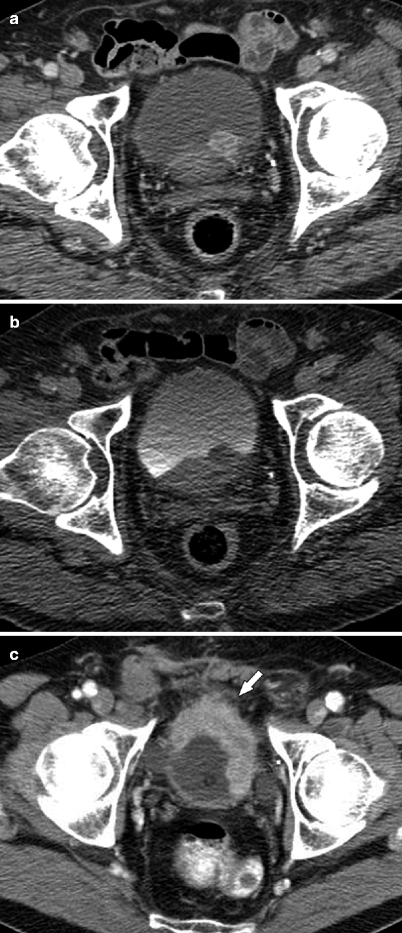
Fig. 3.4
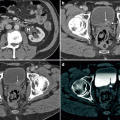
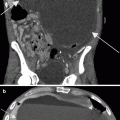
CT illustrations of urothelial carcinoma. Primary tumor may manifest as intraluminal papillary or nodular mass, or plaque-like wall thickening. Images should be acquired 60–80 s after intravenous contrast administration to optimally show tumor enhancement [29]. Axial CTU shows enhancing intraluminal mass on parenchymal phase image (a) and nodular filling defect on excretory phase image (b). (c) Axial parenchymal phase CTU in a different patient demonstrates enhancing thickening of the anterior and left lateral bladder wall. Associated perivesicular fat infiltration (arrow) is compatible with pathologically proven tumor invasion into adjacent fat
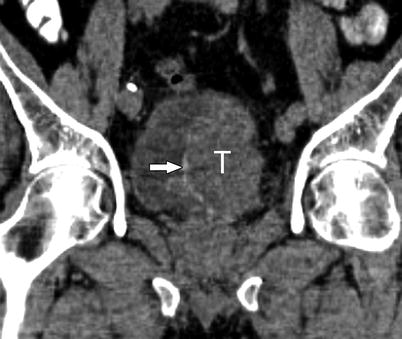
Fig. 3.5
Tumoral calcifications in urothelial carcinoma. Tumoral calcifications may be seen on CT in approximately 5 % of urothelial carcinoma [30]. Calcifications typically encrust the surface of the tumor. Coronal noncontrast CT shows large left intraluminal bladder mass (T) with thin peripheral calcifications (arrow)
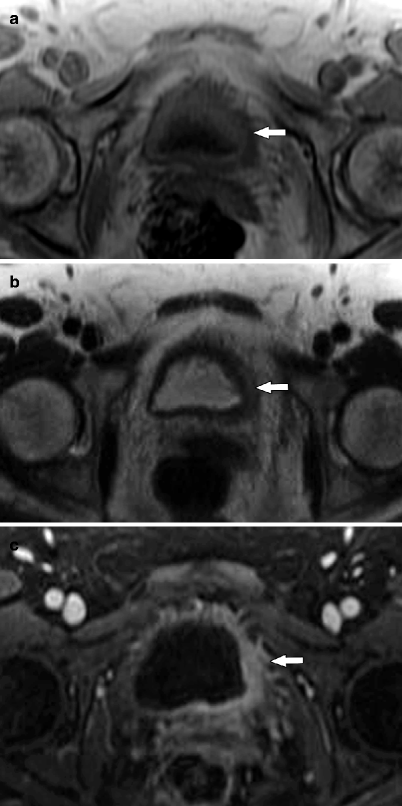
Fig. 3.6
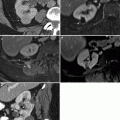
MR illustration of urothelial carcinoma. Urothelial carcinoma is usually isointense to muscle on T1-WI, isointense to slightly hyperintense to muscle on T2-WI, and enhances earlier and more avidly than normal bladder wall or post-biopsy changes such as edema or fibrosis [31]. (a) Axial T1-WI shows a small mass with SI similar to that of detrusor muscle (arrow). Therefore, it is poorly delineated on this sequence. (b) Axial T2-WI demonstrates focal wall thickening that is mildly hyperintense to detrusor muscle (arrow). (c) Axial CE T1-WI image illustrates left lateral and posterior bladder wall thickening (arrow) that enhances greater than normal right lateral bladder wall
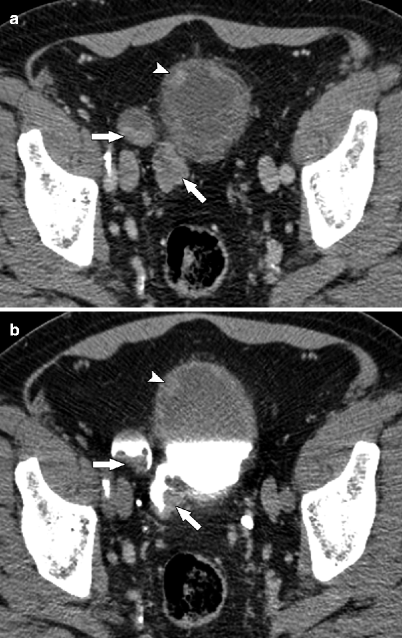
Fig. 3.7
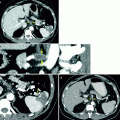
Diverticular urothelial carcinoma. Cross-sectional imaging is useful in detecting neoplasms arising in bladder diverticula, especially when they cannot be accessed at cystoscopy. Bladder diverticula are associated with increased risk of developing bladder cancer (2–10 %), especially urothelial carcinoma, because of urine stasis [32]. Diverticular urothelial carcinoma has worse overall prognosis because of absent muscle layer in the diverticular wall and early tumor invasion into perivesicular fat. (a, b) Axial parenchymal and excretory phase CTU images show urothelial carcinomas (arrows) within bladder diverticula. Additional small intraluminal mass is seen in the anterior bladder wall (arrowhead) (Image courtesy of Dr. Robert Lefkowitz, M.D.)
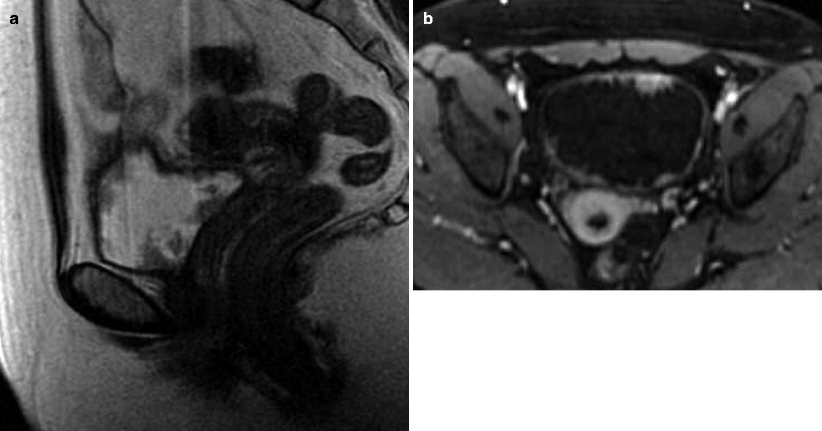
Fig. 3.8
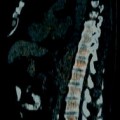
Multicentric urothelial carcinoma. Urothelial carcinoma has a tendency to be multicentric with synchronous and metachronous bladder and upper tract lesions. Multiple bladder tumors are seen in up to 30–40 % of cases [33]. Upper tract tumors occur in 3–5 % of bladder tumor cases and are more common in the setting of multiple bladder lesions [34, 35]. (a, b) Sagittal T2-WI and axial CE T1-WI demonstrate multiple enhancing intraluminal papillary lesions consistent with synchronous low grade non–muscle-invasive papillary carcinomas
Teaching Points |
Urothelial carcinoma is by far the most common bladder tumor. |
Urothelial carcinoma has a tendency to be multicentric with synchronous and metachronous bladder and upper tract lesions. |
It is critical to evaluate the entire urothelium. |
Imaging Appearance of Other Select Epithelial Bladder Neoplasms
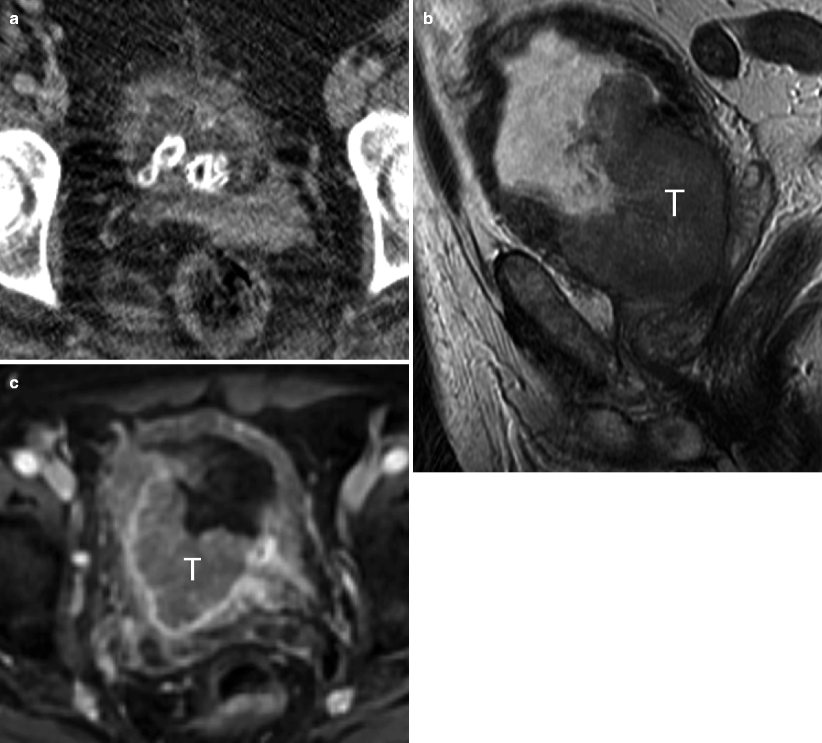
Fig. 3.9
Squamous cell carcinoma. SCC accounts for 5 % of bladder cancers in the United States but is responsible for more than 50 % of bladder tumors in parts of the world where schistosomiasis is endemic [32, 36]. The imaging appearance of SCC is nonspecific. Bladder wall thickening and calcifications from chronic inflammation and/or infection (especially in the setting of schistosomiasis) frequently coexist and make diagnosis more difficult. Muscle invasion is common (about 80 % of cases) and extensive extravesical spread into adjacent organs may be present [37]. (a) Axial CE-CT shows partially decompressed urinary bladder with multiple intraluminal calculi, thickened bladder wall due to biopsy-proven SCC, and mild perivesicular fat infiltration from chronic inflammation. (b, c) Sagittal T2–WI and axial CE T1–WI demonstrate large tumor (T) with invasion into perivesicular fat, prostate gland, and right seminal vesicle
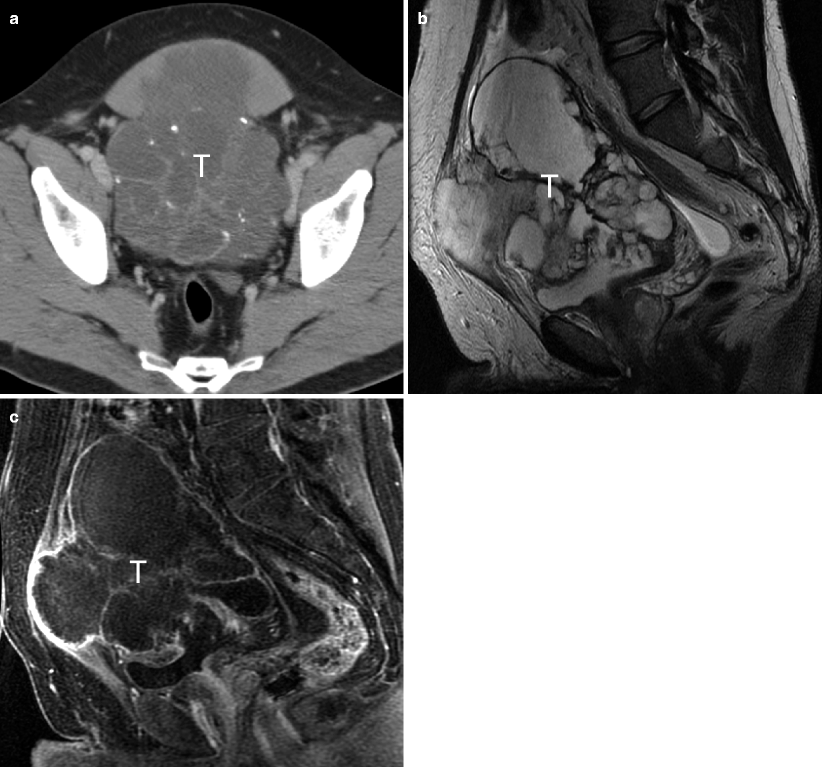
Fig. 3.10
Urachal adenocarcinoma. Adenocarcinomas are rare tumors and represent less than 2 % of bladder cancers. They are subdivided into metastases, as well as primary bladder, and urachal neoplasms. Metastatic adenocarcinoma to the bladder is more common than primary bladder adenocarcinoma. Metastases can invade the bladder by direct extension from other pelvic organs such as the colon, rectum, or prostate gland. Alternatively, metastases may involve the bladder via hematogenous or lymphatic spread from breast, lung, or stomach [38]. Primary adenocarcinomas may be histologically identical to adenocarcinoma of the colon. On CT, diffuse bladder wall thickening and perivesicular fat stranding are common imaging features. Unlike urothelial carcinoma, adenocarcinomas have propensity for peritoneal metastases [39




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree