(1)
Department of Medical System Engineering and School of Mechatronics, Gwangju Institute of Science and Technology, Gwangju, South Korea
Abstract
Only a few techniques are currently available for quantifying systemic metastases in preclinical models. Cancer cell expression of naturally secreted Gaussia luciferase (Gluc) provides a useful circulating biomarker that enables the monitoring of metastatic tumor burden and of treatment response from minimal drops of blood. This blood-based Gluc assay exhibits several distinct advantages: (1) It is highly sensitive in quantifying metastatic tumor growth, particularly when compared to whole-body bioluminescence imaging (BLI) alone; (2) It is quantitative by nature and reflects viable tumor burden in a minimally invasive manner; (3) Through longitudinal collection of blood samples, treatment response can be monitored in real-time; and (4) Gluc bioluminescence provides a means to localize and assess metastatic colonization using BLI. By elucidating the progression of systemic metastases and therapeutic response in animal models, the blood-based Gluc assay is emerging as a valuable quantitative tool for novel drug development.
Key words
BiomarkerGaussia luciferaseBioluminescenceTreatment response monitoringTumor metastasisBlood-based assay1 Introduction
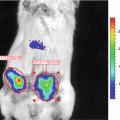
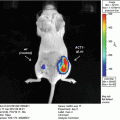
The evaluation of metastatic tumor burden in animal models is not trivial. Oftentimes, metastasis can only be assessed at the sacrificial end point while the burden during disease progression remains unknown. This challenge is particularly problematic for preclinical therapeutic studies, where it is important to control for the tumor burden at the start of any treatment. Currently, bioluminescence imaging (BLI), paired traditionally with firefly luciferase (Fluc) as the reporter, is used to localize tumors and to evaluate metastatic tumor growth in vivo. Although BLI is a powerful tool in itself, the optical signal from Fluc, as well as those of other optical reporters, provides only a semi-quantitative measurement of metastatic tumor burden. A highly sensitive quantitative assessment is hindered not only by the relatively poor spatial resolution of BLI due to light scattering but also by the attenuation of an optical signal traveling through living tissue. These limitations make tumors located deep inside tissue appear very different than their true size, thereby inaccurately reflecting the burden of disease. For small metastatic cell clusters, evaluation becomes even more difficult, if not impossible [1]. For these reasons, it is impractical to employ BLI as a strict quantitative comparison of tumor burden between different experimental animals.
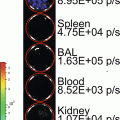
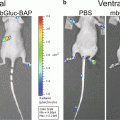
Secreted reporters in the blood have emerged as promising tools for the detection, quantification, and noninvasive monitoring of biological processes in experimental models [2–11]. Tumor cell expression of the naturally secreted Gaussia luciferase (Gluc), derived from the marine copepod Gaussia princeps, has been demonstrated as a sensitive and quantitative method for evaluating cancer progression in vivo [2, 3, 11–13]. Gluc exhibits advantages over other commonly used reporters for BLI. It is 20,000-fold more sensitive than secreted alkaline phosphatase and approximately 2,000-fold more sensitive than Fluc or Renilla luciferase (Rluc), although this measure may vary due to differences in peak emission wavelength (480 nm for Gluc; 612 nm for Fluc) and the depth of a particular tumor from a tissue surface [5, 14]. Furthermore, since Gluc is a secreted enzyme, its concentration in bodily fluids (i.e., whole blood, urine, plasma, etc.) correlates with its expression by a given biological system, thereby quantitatively reflecting the amount of viable cancer cells in primary and metastatic tumors [2, 3, 12]. The blood-based Gluc assay is highly sensitive and provides a novel means to longitudinally monitor metastatic progression as well as treatment response [2, 11].
Application of the Gluc reporter confers additional advantages. When tumor growth is at a stage that is not yet identifiable by BLI, the blood-based Gluc assay facilitates the detection of such early systemic metastasis. Also, Gluc acts on a different substrate (i.e., coelenterazine) than Fluc (i.e., d-luciferin) for bioluminescence; if the reporters are co-expressed, this enables simultaneous monitoring of both tumor burden and signaling pathway activity [15, 16]. As a whole, the quantitative nature of the Gluc reporter and the experimental capabilities have made Gluc assay technology an emerging tool in cancer research. It improves the study of tumor metastasis and aids in the development of strategies for treating this devastating disease.
2 Materials
2.1 Gluc Transfection
1.
2.
Dulbecco’s modified Eagle Medium (DMEM) with 10 % Fetal bovine serum (FBS) and 1 % penicillin–streptomycin (P/S) solution.
3.
4.
Polybrene ((hexadimethrine bromide).
2.2 Experimental Metastasis Model (Intracardiac Injection Model)
1.
Cell strainer (40 μm pore size).
2.
Phosphate Buffered Saline (PBS).
3.
6–7-week-old female nude mice.
4.
Anesthetics.
5.
30 g needle.
2.3 Blood/Urine Gluc Assay
1.
50 mM EDTA (Ethylenediaminetetraacetic acid).
2.
Scalpel.
3.
Coelenterazine (CTZ, Nanolight, Pinetop, AZ).
4.
Methanol mixed with few drops of HCl (hydrogen chloride) for reconstitution of CTZ.
5.
5 mM NaCl, pH 7.2.
6.
Opaque (white or black) 96-well plate.
7.
Plate luminometer (a Centro XS, Berthold Technologies, Oak Ridge, TN was used for bioluminescence imaging).
2.4 Bioluminescence Imaging
1.
IVIS Imaging System (Lumina II, Caliper Life Sciences, Hopkinton, MA) was used for BLI recording.
2.
Living Image software 3.0 coupled to the IVIS system.
3.
50 U insulin syringe (26 g needle).
3 Methods
3.1 Gluc Transfection
1.
Culture 231BR cells in a 6-well plate in DMEM with 10 % FBS and 1 % P/S (10 % DMEM) at 37 °C.
2.
When cells have grown to 60–70 % confluency, replace the medium with 1 ml of 10 % DMEM containing lentivirus at a multiplicity of infection of 50 and with 1 μl of 8 mg/ml polybrene. Continue cell culture for 24 h (see Note 3).
3.
Replace the lentiviral medium with 10 % DMEM for continued cell culture.
4.
After 2–3 days, check CFP (cerulean fluorescent protein) expression using fluorescence microscopy. If fluorescence is faint, repeat the transfection with the same dose of lentivirus (see Note 4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree