3
Bone Metastases
Pei Shuen Lim and Peter J. Hoskin
INTRODUCTION
Approximately 50% of the workload in a radiation therapy (RT) department is comprised of palliative work, of which the most common indication is bone metastases.1 Bone is the third most frequent site of metastatic disease following lung and liver.2 The incidence of bone metastases is highest in breast and prostate cancer, where up to 80% of patients have evidence of metastatic bone disease at autopsy studies.3 Other tumors that frequently metastasize to the bones include lung, kidney, and thyroid cancers, with an incidence of around 30%.4
Bone metastases affect the axial skeleton more commonly than the appendicular skeleton. It has been suggested that the reason for this is that the axial skeleton contains a higher proportion of red marrow, which has an increased blood supply, stem cells, and extracellular matrix, therefore promoting tumor growth. The distribution of bone metastases in the skeleton has been described in this descending order of frequency: lumbar spine, thoracic spine, pelvis, ribs, femur, skull, cervical spine, humerus.2 In the long bones, proximal regions are involved earlier than distal regions.
Symptoms from bone metastases can cause significant morbidity, affecting the patient’s overall quality of life and function. Local symptoms include pain, pathological fracture, spinal cord compression, and nerve root compression. Systemic complications can also occur such as hypercalcemia and bone marrow suppression secondary to marrow infiltration. The main goals of palliative treatment in this setting are pain relief and function preservation.
RADIATION THERAPY FOR BONE METASTASES
Local Bone Pain
External beam radiation therapy (EBRT) offers excellent local palliation for painful bone metastases leading to an improvement in quality of life with little toxicity.5,6 The median time to onset of pain relief is around 3 weeks.7 Overall pain relief is obtained in 60% to 80% of patients and 25% of patients achieve complete response after initial EBRT.8 The median duration of response varies between 11 and 24 weeks.9 The net pain response is an objective method of assessing duration of pain relief relative to the survival of a patient, which has been found to be between 63% and 71%.10,11
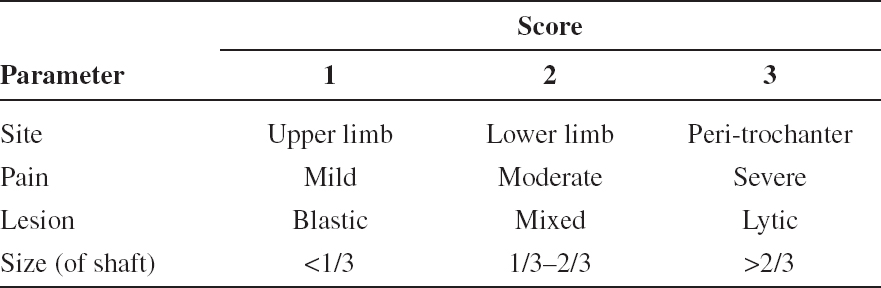
TABLE 3.1 Mirels’ Score for Assessing Risk of Pathological Fracture. A Total Score of Greater Than 8 Suggests the Need for Prophylactic Internal Fixation Prior to Radiation Therapy12
Pathological Fracture
Metastases weaken the stability of the bone, leading to an increased risk of pathological fracture especially in weight bearing bones. The morbidity and mortality of a fracture are significant. Therefore, accurate prediction of the risk of an impending pathological fracture is crucial—not only to prevent the morbidity of a fracture in high-risk patients but also to avoid overtreatment with unnecessary prophylactic osteosynthesis in a group of patients with limited life expectancies, who may benefit from simple RT or bisphosphonates if the lesions are at lower risk of fracturing.
The Mirels’ score is based on four criteria: pain, size, site, and nature, to form a 12-point scale to predict the risk of impending fracture (Table 3.1).12 This scoring system has been found to overestimate the risk in some studies.13,14 The Dutch Bone Metastases study group recommends the use of a simple radiographic parameter measuring the largest axial cortical involvement (Lcort).15 If the Lcort is less than 30 mm, RT is recommended. If the Lcort is more than 30 mm, prophylactic surgical osteosynthesis should be considered followed by RT if appropriate.
DOSE FRACTIONATION
Single fraction (SF) and multiple fraction (MF) RT schedules for the treatment of uncomplicated bone metastases have been shown to be equivalent. Four large meta-analyses, including an update in 2012, reviewing multiple randomized controlled trials has consistently confirmed this result (Table 3.2). Overall and complete response rates were similar. No additional benefit was seen with higher total doses of RT regardless of tumor type, expected survival, or site of metastases. Although retreatment rates with SF seem to be higher at 20% compared to 8% after MF, these differences are subject to bias as the option of retreatment was at the discretion of the treating clinician.
TABLE 3.2 Summary of the Outcomes and Adverse Events Shown in Meta-Analyses of Randomized Clinical Trials Comparing Single Fraction (SF) Versus Multiple Fraction (MF) Radiation Therapy Regimens

SF RT is convenient, efficient, and effective.18 It reduces unnecessary hospital visits, particularly benefiting patients who are facing end of life, are limited by pain, or have poor performance status. It is also cost effective for a RT department, allowing appropriate utilization of resources.19,20
The American Society for Radiation Oncology (ASTRO)6 and American College of Radiology (ACR)21,22 have published treatment guidelines for bone metastases recognizing the benefits of SF. 8 Gy has been found to be the optimal SF dose in achieving response when randomized against 4 Gy SF.23 Recommended MF schedules by ASTRO are 30 Gy in 10 fractions, 24 Gy in 6 fractions, and 20 Gy in 5 fractions.
In 2012, the National Quality Forum (NQF), a U.S.-based organization that reviews quality metrics, has endorsed a performance measure on the use of EBRT for bone metastases based on the ASTRO guidelines, supporting shorter RT schedules.24 This measure is aimed at addressing the gap in dose fractionation treatment variations among clinicians25 and preventing the overuse of radiation therapy. The “Choosing Wisely” campaign is an initiative targeted at reducing unnecessary tests and treatments in the U.S. health care system. They have recommended the use of SF and discouraged the routine use of extended fractionation schedules (>10 fractions) for the palliation of bone metastases.26,27
Despite extensive evidence supporting the use of SF and published guidance, international surveys still show that SF is still underutilized globally.25,28 One reason against the use of SF is the higher retreatment rates associated with it.29 The trial protocols left the option to retreat up to clinician discretion. They were not blinded to initial dose fractionation schedules, leading to biased results. Clinicians were more likely to offer retreatment after SF RT as they would still be within radiation tolerance after SF and there is the possibility that clinicians may have felt that SF treatment was insufficient.30
Some argue for the use of MF in order to achieve longer term responses in patients with better prognoses. A subgroup analysis of patients with more favorable prognoses, surviving more than 1 year, in the Dutch Bone Metastases study demonstrated no additional benefit in the duration of response after initial response with MF compared to SF, which was around 29 to 30 weeks in both arms.31 Oncologists in general tend to be more optimistic in predicting survival.32,33 It is important to remember that symptomatic pain relief is the main goal of treatment for the majority of patients with metastatic bone disease, not tumor control. For patients with true oligometastatic disease and good performance status, MF or stereotactic RT may be a reasonable option.
For neuropathic pain, only a single trial so far has addressed fractionation.34 The authors concluded that SF was neither as effective, nor worse than MF. The duration of response was slightly better after MF. Further trials in this area should therefore be carried out to validate these results.35
Higher total doses, 20 to 30 Gy, are considered appropriate for complicated bony lesions with extensive soft-tissue components or lesions with impending fractures, which are surgically inoperable.36 Increased tumor control with higher doses allows the induction of bone mineralization. However, this process may take up to several months to occur. It is therefore imperative to carefully select patients who are offered MF to ensure that the clinical benefits are not offset by the side effects and prolonged inconvenience of fractionated treatment.
REIRRADIATION
Improvements in systemic and supportive therapies have increased the life expectancies of patients with bone metastases. More patients are therefore outliving the duration of benefits from their initial RT. Approximately 50% of patients who responded to initial treatment experience pain relapse within 1 year from treatment.7
Two systematic reviews have confirmed the efficacy of reirradiation of painful bone metastases.37,38 Repeat radiation is indicated for patients in these three scenarios:
1. No pain relief after initial radiation
2. Partial response after initial radiation who may receive benefit from further doses
3. Pain relapse after satisfactory response to initial radiation
It is recommended that a 4-week period should be given before considering reirradiation to allow enough time to observe the response from the initial treatment.8
The NCIG CTG SC20 is a prospective randomized trial that investigated dose fractionation schedules of reirradiation. 8 Gy SF was found to be noninferior and less toxic compared to 20 Gy MF.39 The overall response rate for reirradiation was 48% regardless of response to initial therapy, with 11% to 14% achieving complete response in the per-protocol analysis. Sixty-eight percent of patients reported an improvement in their quality of life.
A pooled analysis looking at overall response rates in patients who underwent both initial treatment and retreatment demonstrated that there was no difference in outcome between different fractionation schedules: SF/SF, SF/MF, MF/SF, and MF/MF. All schedules had overall response rates within 2% of each other.40
When retreating spinal metastases, spinal cord tolerance must be considered. Cord tolerance is still a subject of uncertainty. The risk of myelopathy is low if the interval between treatments is no less than 6 months and the total cumulative biological effective dose (BED) is kept below 135.5 Gy2 (α/β = 2).41 QUANTEC recommends using cord α/β at 0.87 and estimates a 0.2% risk of myelopathy at 50 Gy EQD2. Cord recovery is at least 25% at 6 months after initial treatment.42 A dose of 30 Gy in 10 fractions delivers 40.6 Gy EQD2, 20 Gy in 5 fractions delivers 34 Gy EQD2, and an 8 Gy SF delivers 24.8 Gy EQD2. (EQD2 formula = (n·d [1 + d/α/β])/1 + 2/α/β; n = number of fractions, d = dose per fraction.) This illustrates how a higher dose given at initial treatment may preclude the patient from being offered retreatment at a later date, as it may exceed cord tolerance.
CHOICE OF TREATMENT AND PROGNOSIS
Patients with painful bone metastases have a median survival of 7 to 9 months.31
Even in the group of patients who were reirradiated in the SC20 trial, median overall survival was between 9.3 and 9.7 months, which is likely to be an overestimate of the unselected population.39 Survival is dependent on type of primary tumor, with breast and prostate cancer extending over years, while lung cancer is measured in months.4,43 The presence of visceral metastases is associated with a worse prognosis.
With no strong evidence advocating one schedule over another, the choice of treatment needs to be made together with the patient and her family, taking into account symptoms, life expectancy, quality of life, cost, and expectations. An initial SF treatment followed by a repeat SF if necessary is a more pragmatic and cost-effective approach than upfront MF RT. The cost of two SF treatments is lower than MF, even after taking into account the additional costs of medications and hospital admissions.20,44
TREATMENT PLANNING
General Radiation Therapy Planning and Delivery
The following is a stepwise approach in the work up of delivering RT to bone metastases:
![]() Gather and interpret all relevant clinical information
Gather and interpret all relevant clinical information
![]() History, examination, and assessment—Where is the pain if there are multiple sites of bony metastases on imaging? What stage of the disease is the patient at? Are there any systemic treatment options available? What is his performance status?
History, examination, and assessment—Where is the pain if there are multiple sites of bony metastases on imaging? What stage of the disease is the patient at? Are there any systemic treatment options available? What is his performance status?
![]() Diagnostic imaging—MRI, CT, bone scan, plain radiographs
Diagnostic imaging—MRI, CT, bone scan, plain radiographs
![]() Consent
Consent
![]() Simulation
Simulation
![]() Fluoroscopy or CT planning
Fluoroscopy or CT planning
![]() A conventional fluoroscopy simulator is being replaced with CT simulation in most departments as CT images allow optimal anatomical visualization of target volumes and critical structures. Virtual simulation is adequate for straightforward cases. Formal planning with dose distribution assessment may be beneficial for tumors that are in close proximity to organs at risk, such as large para-spinal masses, where there may be an increased risk of toxicity with large doses per fraction.
A conventional fluoroscopy simulator is being replaced with CT simulation in most departments as CT images allow optimal anatomical visualization of target volumes and critical structures. Virtual simulation is adequate for straightforward cases. Formal planning with dose distribution assessment may be beneficial for tumors that are in close proximity to organs at risk, such as large para-spinal masses, where there may be an increased risk of toxicity with large doses per fraction.
![]() Patient positioning
Patient positioning
![]() The patient should be placed in a comfortable, reproducible position that can be maintained for at least 10 to 15 minutes. The need for adequate analgesia must be anticipated and given.
The patient should be placed in a comfortable, reproducible position that can be maintained for at least 10 to 15 minutes. The need for adequate analgesia must be anticipated and given.
![]() Most sites can be treated supine, with exceptions being the ribs, clavicle, and skull when treated with direct electrons; it may be necessary to be positioned semisupine, lateral, or prone.
Most sites can be treated supine, with exceptions being the ribs, clavicle, and skull when treated with direct electrons; it may be necessary to be positioned semisupine, lateral, or prone.
![]() Arm positioning
Arm positioning
![]() Thorax—above head or by sides depending on the angle of beam entry
Thorax—above head or by sides depending on the angle of beam entry
![]() Pelvis—arms by sides or across chest if using more than two fields
Pelvis—arms by sides or across chest if using more than two fields
![]() Humerus—arms abducted, elbows flexed, hands on hips
Humerus—arms abducted, elbows flexed, hands on hips
![]() Immobilization to aid reproducibility
Immobilization to aid reproducibility
![]() Leg or ankle stocks, headrest, foam wedges
Leg or ankle stocks, headrest, foam wedges
![]() Shoulder retractors can be used to bring shoulders down and away from the lateral fields used to treat the cervical spine region
Shoulder retractors can be used to bring shoulders down and away from the lateral fields used to treat the cervical spine region
![]() Thermoplastic shells can be used for head and neck immobilization
Thermoplastic shells can be used for head and neck immobilization
![]() Immobilization tools used should be kept as simple and practical as possible to allow seamless reproducibility, as most of these patients are in pain and would not tolerate elaborate set-up sessions
Immobilization tools used should be kept as simple and practical as possible to allow seamless reproducibility, as most of these patients are in pain and would not tolerate elaborate set-up sessions
![]() Target volume definition
Target volume definition
![]() Treatment volumes should be based on symptoms, not just radiological findings.
Treatment volumes should be based on symptoms, not just radiological findings.
![]() Field edges represent the 50% isodose line.
Field edges represent the 50% isodose line.
![]() Symptomatic lesions should be adequately included within the 90% isodose region, taking into consideration internal organ movement and set-up variation. A 1- to 2-cm margin is generally sufficient for this purpose.
Symptomatic lesions should be adequately included within the 90% isodose region, taking into consideration internal organ movement and set-up variation. A 1- to 2-cm margin is generally sufficient for this purpose.
![]() Field edges should ideally coincide with anatomical borders to allow for subsequent matching of adjacent fields if future treatments are required. Borders commonly used are the intervertebral spaces, vertebral body, transverse processes, pubic symphysis, lesser trochanter, and acetabulum.
Field edges should ideally coincide with anatomical borders to allow for subsequent matching of adjacent fields if future treatments are required. Borders commonly used are the intervertebral spaces, vertebral body, transverse processes, pubic symphysis, lesser trochanter, and acetabulum.
![]() Structures such as joint spaces and vertebral body should be fully covered.
Structures such as joint spaces and vertebral body should be fully covered.
![]() Balance between disease treated and toxicity should always be considered.
Balance between disease treated and toxicity should always be considered.
![]() Dose prescription
Dose prescription
![]() Single applied fields prescribed to a particular depth or parallel opposed fields prescribed to the midplane dose (MPD) using 6-10-MV photons.
Single applied fields prescribed to a particular depth or parallel opposed fields prescribed to the midplane dose (MPD) using 6-10-MV photons.
![]() For 3D conformal plans, dose is prescribed to 100% at the International Commission on Radiation Units and Measurements (ICRU) reference point.
For 3D conformal plans, dose is prescribed to 100% at the International Commission on Radiation Units and Measurements (ICRU) reference point.
![]() For superficial lesions (ribs, scapula, skull), direct electrons or orthovoltage beams can be used.
For superficial lesions (ribs, scapula, skull), direct electrons or orthovoltage beams can be used.
![]() Verification and image guidance
Verification and image guidance
![]() This step is important especially with hypofractionated schedules. KV portal imaging or cone beam CT can be used to verify patient set up and guide positional corrections prior to treatment delivery.
This step is important especially with hypofractionated schedules. KV portal imaging or cone beam CT can be used to verify patient set up and guide positional corrections prior to treatment delivery.
RADIATION THERAPY PLANNING TO THE SPINE
The whole vertebra containing the lesion should be treated. One vertebra superior and inferior to the involved site should be included, with field edges at the intervertebral space. This ensures the vertebra intended for treatment is adequately covered within the 90% isodose level as the field borders represent the 50% isodose. Lateral borders are the transverse processes and should encompass any paravertebral disease extension.
Several cases and figures are presented to illustrate radiation planning for bone metastases.
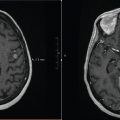
Case 3.1: Upper Cervical Spine Radiation Therapy