Fig. 1
Several devices used to create a plane radiating surface for cutaneous and mucosal lesions. Each type of applicator could differ in size, shape, and radium content depending on the lesion being treated [6]. (a) Flat radium applicator with removable handle attached to a radium plaque. (b) Flat radium applicator embedded in dental compound and arranged for treatment of a lesion on the dorsal surface of the tongue. (c) Three tubular applicators attached to a flat wooden device. Tubular applicators could be filled with radium or radon gas. (d) Same as part (c), covered in heavy rubber. Filtration of this device was achieved by applying a thin metallic screen to the tubes and then covering the device with rubber to remove secondary rays
Modern interstitial and surface-mold brachytherapy techniques, which will be discussed in a later section, have been modeled after techniques that were developed and used successfully during this period. For easily accessible lesions that warranted superficial therapy, radium plaques, radium tubes, or radon tubes were applied directly to the tumor or a short distance from it using a variety of devices. For irregular or inaccessible cutaneous or mucosal lesions, it was customary to make a mold or cast of the tumor lesion using dental compound. Either radium element applicators or radon emanation tubes were then applied to this mold, creating a plane radiating surface directly over the tumor (Fig. 1). Where intralesional (interstitial) therapy was indicated, tiny glass or gold radon seeds were most often permanently implanted into malignant tissue (Fig. 2) [6]. After about 1930 radium puncture became the preferred technique, in which 2–7 radium needles loaded with 5–10 mg of radium were applied to the surface of the tumor at intervals of 5 mm for 4–5 h. At that time, the 10-year control rates with direct contact therapy and radium puncture were reported to be 73.8 % and 84 %, respectively [1].
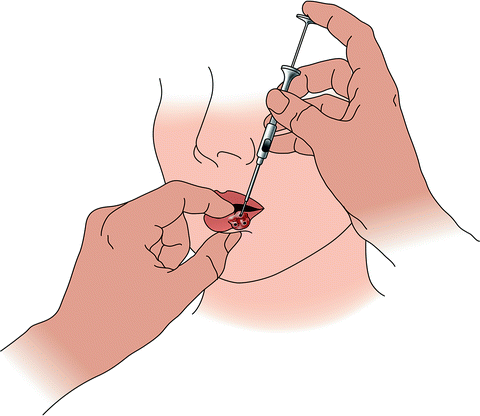

Fig. 2
Method of inserting radon seeds into a carcinoma of the lower lip, as performed in the 1920s [6]. Each seed was pushed through the embedding needle and into the tissue, where it was left permanently in situ
However, despite the growth and advancement of brachytherapy techniques during this period, by the mid-1940s the popularity of brachytherapy as a modality for the treatment of skin lesions began to decline as it was gradually replaced by X-ray therapy [7, 8]. X-rays had become easier and more convenient to employ and at least as equally efficient as radium in the majority of cases. In the following decades, brachytherapy continued to be utilized in dermatology, but the indications became much more limited. It remained the treatment of choice for areas that poorly tolerate irradiation such as the dorsum of the hand and foot, as well as for patients who were unable to leave their homes for treatment, but it lost ground in most other areas [8].
Notably though, the field of brachytherapy has continued to advance, primarily because it has remained a preferred treatment modality for other types of malignancies [9]. After World War II, many new artificial radionuclides became available, most notably cesium-137 (137Cs), iridium-192 (192Ir), and iodine-125 (125I), which carried certain advantages over radium [2, 10]. In addition, since the mid-1960s, radioactive sources are no longer implanted directly into the patient for safety reasons. This practice exposed the radiation oncologist and staff to unacceptable levels of irradiation, so instead nonradioactive applicators such as tubes or catheters are first implanted into the target site, and then radioactive sources are “afterloaded” into this apparatus. In the 1970s, manual afterloading was replaced by remote afterloading, which allows the operator to remain in a shielded site and eliminates all exposure for medical personnel [1, 10]. Later, high-dose-rate (HDR) units were introduced as opposed to the already widely used low-dose-rate (LDR) units. HDR permits the radioactive source to be removed between treatments, making short sessions of irradiation possible on an outpatient basis, without any undue risk of irradiation to the patient or medical staff [2]. More recently, brachytherapy has significantly benefited from further studies regarding dosimetry as well as the use of advanced imaging technologies, which have enabled optimization of treatment planning, dose distribution, and clinical outcomes [9, 10].
Today, brachytherapy is a mainstay of treatment for prostate, breast, cervical, endometrial, and certain head and neck cancers [1, 9]. Teletherapy, also called external beam radiation (see Chap. 12), remains the most commonly used radiation modality for skin cancer in the United States [11, 12], but brachytherapy continues to be a widely accepted treatment option for specific nonmelanoma skin cancer lesions. Furthermore, due to the important recent advances in the field mentioned above, brachytherapy has been gaining popularity again [13], and use for skin cancers is gradually increasing worldwide [14].
Technical Aspects
Brachytherapy has consistently provided a highly conformal radiation therapy modality vs. other radiation methods [9]. Because all brachytherapy techniques involve placement into or onto the target tissue, the normal tissue outside the radiation zone receives a negligible radiation dose. This minimizes unwanted dose delivery to nearby radiation-sensitive tissues such as the brain or bone, and ensures the best chance of prompt healing and the smallest chance of late radiation morbidity. Thus, brachytherapy can be performed on the scalp and other areas of the body where traditional external radiotherapy may be less safe [1].
Modes of Administration
In modern brachytherapy, placement of the radioactive source may be on the body surface (surface-mold technique), into body tissues (interstitial), into a body cavity (intracavity), or across a tissue boundary into a contained space (transluminal) [1]. Both the surface-mold technique and interstitial brachytherapy have been used in the treatment of skin cancer.
The surface-mold technique utilizes custom molds that are created from impressions of the tumor surface. Molds are constructed from pliable materials, such as silicone or polymethyl-methacrylate, and are then fitted with radioactive isotopes and applied directly to the tumor [15]. Radioactive sources are loaded into the mold in such a way as to distribute uniform dosage throughout the tumor volume. Surface-mold brachytherapy is most often used for the treatment of well-circumscribed, superficial tumors [1].
Interstitial brachytherapy constitutes an invasive means of internal radiation therapy in which radioactive wires or seeds are placed directly within the tissue at the target site. Interstitial brachytherapy is well suited for certain areas, such as the eyelid, where the creation of the precise surface mold required for surface-mold brachytherapy is technically unfeasible [1].
Radiation Source and Dose Delivery
Interstitial brachytherapy implants may be permanent or temporary [16]. Permanent brachytherapy implants emit radiation at very-LDR that is equivalent to less than 0.4 Gy/h for the lifetime of the radioactive isotope. Typically, 125I is utilized in permanent implants because of its emission of relatively low mean energies [1].
On the other hand, temporary implants are associated with greater variation in dose rates (Table 1). LDR implants deliver 0.4–2 Gy/h over durations from 24 to 144 h in an inpatient setting. Medium-dose-rate (MDR) devices deliver 2–12 Gy/h and HDR devices deliver greater than 12 Gy/h [17]. The most commonly used isotope for temporary implantation is 192Ir. 192Ir has a half-life of 74.2 days and emits γ rays with a mean energy of 380 keV. Other γ-emitting isotopes used for temporary implantation include cobalt and cesium, especially in the past [1].
Table 1
Comparison of dose delivery with common brachytherapy techniques
LDR 192Ir | MDR | HDR | |
|---|---|---|---|
Dose rate | Low | Medium | High |
Duration per treatment | 2–6 days | 1 day | Minutes |
Duration of treatment course | 2–6 days | 1 day | 3–5 weeks |
Availability (internationally) | ++ | − | − |
Ease of optimization | − | − | + |
Dose per treatment, Gy | 60 | 40 | 0.18–0.7a |
No. of fractions | 1 | 1 | 7–35 |
Total dose as sole modality, Gy | 60 | 40 | 35–50a |
Surface-mold brachytherapy is often delivered using HDR units, but has also been studied using LDR units. 125I has been used for LDR surface-mold brachytherapy, while most studies on HDR surface-mold brachytherapy have evaluated the use of the γ-emitting 192Ir [1]. Notably, at least two studies on surface-mold brachytherapy have explored the use of a mixed β-γ isotope, either rhenium-188 or holmium-166 [18, 19].
A single treatment of LDR brachytherapy can span 3–5 days, requiring hospitalization for the patient and radiation protection for individuals who are in contact with the patient for the duration of treatment. Alternatively, a HDR brachytherapy treatment can be completed in 1–30 min, usually in the outpatient setting. However, HDR brachytherapy is more likely to cause damage to surrounding healthy tissue than is LDR therapy. To prevent such complications, the total dose of HDR brachytherapy is commonly divided into a few or as many as 30–40 sessions, occurring every 1–28 days [1].
Operator Safety
As with all radiotherapy techniques, brachytherapy involves potential risks of radiation exposure to medical personnel involved in treatment. LDR is associated with secondary radiation risk because the duration of treatment is extended for days, and HDR is associated with radiation risk because the radiation source is of high intensity. As mentioned above, remote afterloading techniques are often used to enhance operator safety. Afterloading allows nonradioactive implantation devices, such as flexible plastic or stiff guiding metal tubes, to be placed within the tumor first, and then radioactive sources can be mechanically loaded through these tubes or catheters remotely [1, 2, 10].
Selected Studies Regarding the Use of Brachytherapy
Surface-Mold Brachytherapy
LDR surface-mold brachytherapy has been studied in retrospective studies and case series evaluating the treatment of NMSCs of the face and eyelid (Table 2). In one retrospective case–control study, the cosmetic outcome was compared for 15 patients treated for basal cell carcinoma (BCC) of the face using gold grain Elastoplast molds (Beiersdorf, Birmingham, England) and 15 patients treated for the same indication using fractionated superficial X-ray [20]. The brachytherapy arm tumors received total doses of 60–65 Gy during a 7-day application, and all of the case and control tumors had been treated over 10 years prior. Long-term cosmesis was observed to be superior in the brachytherapy group. This result was attributed to the rapid decrease in brachytherapy radiation dose beyond the superficial tissues. It was also hypothesized that X-ray treatment may be relatively more likely to induce very late skin and subcutis adverse effects than brachytherapy [1]. One case series described the treatment of several eyelid tumors, including two neglected BCCs on the verge of orbital invasion [21]. LDR brachytherapy was applied using a 15-mm diameter gold shield to which an 125I plaque was affixed on the exterior (eyelid side). The shield served to protect the globe while permitting irradiation of the target eyelid lesion with 50 Gy to a 5 mm depth.
Table 2
Summary of previous studies of brachytherapy for nonmelanoma skin cancer
Study | Site studied | Lesion type | Modality | Treatment time | Dosage (cGy)/fractions | Follow-up (mo) | Recurrence rate |
|---|---|---|---|---|---|---|---|
Avril et al. [27] | Face | BCC (347) | LDR interstitial | 165 h | 5,700–9,600/1 | 48 | 8/95 |
Berridge and Morgan [20] | Face | BCC (30/30) | LDR surface mold | 168 h | 6,000–6,500/1 | 120 (minimum) | Unknown |
Conill et al. [30] | Eyelid | SCC (4/24) | LDR interstitial | 54–55 h (total) | 4,000 | 43 (mean) | 2/24 |
BCC (19/24) Adenocarcinoma (1/24) | |||||||
Conill et al. [29] | Lip | SCC (52/54) | LDR interstitial | 86 h | 6,000–6,500/1 | 96 (mean) | 2/54 |
BCC (2/54) | |||||||
Debois [24] | Nose | Epidermoid (60/370) | HDR surface mold | 48 h (total) | 2,400 | >36 | 11/368 |
BCC (300/370) Other (10/370) | |||||||
Guix et al. [22] | Face | SCC (34/136) | HDR surface mold (117/136) | 3–8 min/session | 6,000–6,500/33–36 (<4 cm) | 60 | 3/136 |
BCC (102/136) | HDR Brock applicator (19/136) | 7,500–8,000/10 (>4 cm) | |||||
Lee et al. [19] | Various |





