Congenital brain malformations comprise a spectrum of disorders that result from a variety of causes, including genetic abnormalities, ischemia, infections, and toxic exposures. Although most cases are discovered in infancy or childhood, clinically occult abnormalities may prove to be confounding, especially if first encountered later in life on imaging examinations obtained for other indications or in the context of superimposed pathology. This review article provides an overview of congenital brain malformations because they may be encountered at all ages for general radiologists.
Key points
- •
Congenital brain malformations often manifest in an imaging spectrum of mild to severe forms with clinical phenotype often corresponding with the imaging findings.
- •
In milder manifestations of brain malformations, affected patients may present as adults with first time seizure, developmental delay, or for other unrelated causes.
- •
Surgery for brain malformations is often directed toward the management of secondary findings, such as hydrocephalus or seizures.
- •
In a patient with history of shunted hydrocephalus or epilepsy surgery, one should look for possible underlying congenital malformations.
Introduction
Congenital brain malformations can be encountered in both pediatric and adult patients and with the improving resolution of fetal MR imaging, they are increasingly being diagnosed in the prenatal period as well. Although most malformations are discovered in infancy or childhood, milder phenotypes may manifest clinically later in life or remain clinically silent until discovered incidentally on imaging obtained for other indications. These malformations may prove to be confounding to a general radiologist, particularly if encountered in the context of superimposed pathology or in the setting of surgical complications.
The etiologies for congenital brain malformations are varied and can result from genetic abnormalities, ischemia, infections, and toxic exposures. Recently, the Zika virus epidemic brought much public attention to the effect of congenital infections on the developing brain and importance of early screening and detection. Although the scope of congenital brain malformations are as varied as the causes, identifying the pattern of malformation may suggest the underlying cause or genetic abnormality. This review article provides an overview of brain malformations because they may be encountered at all ages for general radiologists.
Imaging techniques
Plain Radiography
Skull radiography is still commonly obtained in the setting of suspected nonaccidental trauma in the pediatric population. However, it is no longer considered standard of care for most clinical indications in either children or adults owing to its lack of sensitivity and specificity at the cost of radiation dose, which is not insignificant when multiple views are obtained.
Ultrasound Imaging
Ultrasound examination is the primary modality for imaging the fetal and neonatal brain until approximately 9 to 12 months of age, at which time the closing anterior fontanelle provides a limited acoustic window. In infants and neonates, ultrasound examination can be used to screen for intracranial hemorrhage, hydrocephalus, periventricular leukomalacia, and congenital anomalies.
Ultrasound examination is typically performed via the open anterior as well as mastoid fontanelles through which a series of sagittal, parasagittal, and coronal grayscale images are obtained. The mastoid fontanelle offers an optimal window for evaluation of the fourth ventricle and posterior fossa. Color and spectral Doppler can be performed to evaluate the circle of Willis, superficial cortical veins, venous sinuses, and vascular anomalies, such as vein of Galen malformations.
Advantages of ultrasound examination include real-time diagnostic information; portability with the ability to image infants who may be too unstable to travel to computed tomography scanning/MR imaging, and its low cost, allowing for serial screening examinations. Disadvantages include limited sensitivity for detecting early ischemic changes and limited views of structures located deep or peripheral to the field of view provided by the anterior and mastoid fontanelle acoustic windows, such as the lateral temporal lobes or periphery of the posterior fossa structures, an important site of hemorrhage detection in patients on extracorporeal membrane oxygenation.
Computed Tomography Scans
Multidetector computed tomography scanning offers the advantages of rapid acquisition times; the ability to recreate isotropic, multiplanar, reformatted images, and 3-dimensional reconstructions in various algorithms; and fine osseous detail over MR imaging. These advantages should be heavily weighed against the risks associated with the use of ionizing radiation, particularly in children with chronic conditions, who may undergo multiple diagnostic imaging investigations throughout their lifetime. For both children and adults, dose reduction should be optimized via appropriate collimation of the area of interest; indication-appropriate kilovoltage and milliampere-second parameters; and iterative reconstruction techniques, if available, to further decrease the radiation dose.
MR Imaging
In most circumstances in which imaging of the brain are required, MR imaging is the imaging modality of choice. MR imaging offers excellent soft tissue resolution, spatial resolution, and ability for multiplanar imaging acquisition, all with lack of ionizing radiation. Disadvantages include longer image acquisition times and susceptibility to motion, which may be problematic in the pediatric population, particularly between the ages of 6 months and 4 years of age. For children who may be unable to remain still for MR imaging, sedation may be required.
Individual sequences chosen for an MR imaging protocol vary depending on the clinical indication with standard protocols including sagittal T1-weighted imaging; axial and coronal T2-weighted imaging; axial T2 fluid attenuation inversion recovery (FLAIR) imaging; and axial diffusion-weighted imaging. Pediatric patients at the authors’ institution with suspected structural brain abnormalities are imaged with isotropic T1-weighted FLAIR and magnetization prepared T1-weighted MR imaging at isotropic resolution, which accentuate subtle cortical and white matter abnormalities and beautifully depict gray–white matter interfaces. Gadolinium contrast may be administered to better assess primary and metastatic brain tumors, leptomeningeal disease, intracranial infections, demyelination, and neurocutaneous disorders. However, evaluation of brain malformations typically does not require gadolinium contrast.
Spectrum of brain malformations
Malformations of Cortical Development
Disorders of neuronal proliferation
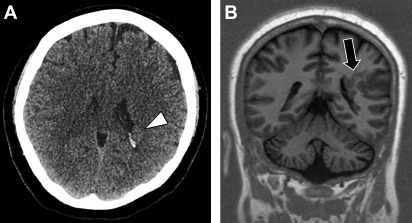
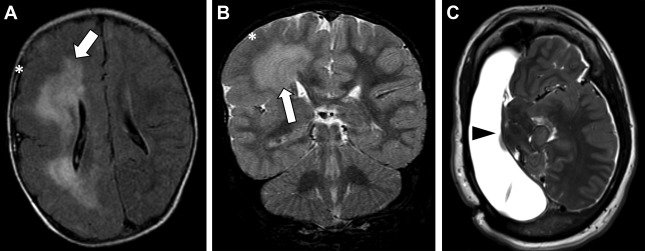
Hemimegalencephaly
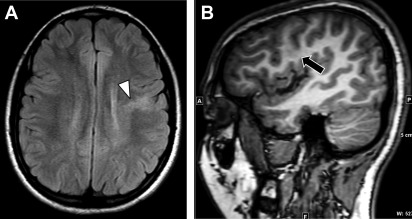
Hemimegalencephaly refers to hamartomatous overgrowth of all or part of a cerebral hemisphere. Often, additional abnormalities such as polymicrogyria, lissencephaly, or gray matter heterotopias are present and may involve the contralateral hemisphere. Clinically, affected patients often present within the first year of life with seizure disorder, macrocephaly, hemiplegia, and profound developmental delay. The incidence of hemimegalencephaly is rare, accounting for 0.2% of children with epilepsy and among those 1% to 14% have malformations of cortical development. If no contralateral malformations are present, seizures are managed with anatomic or functional hemispherectomy, which often requires shunting of the surgical cavity. Surgical shunt malfunctions are common and may present in the adult emergency setting ( Fig. 1 ).
Focal cortical dysplasias
Focal cortical dysplasias are areas of cortical disorganization and abnormal laminar architecture ( Fig. 2 ). They often present in childhood in the setting of intractable epilepsy and are recognized as the most common cause of neocortical pharmacoresistant epilepsy. However, in some instances, focal cortical dysplasias can be clinically occult and are discovered later in life on MR imaging examinations performed for indications other than seizure.
In the setting of medically refractory seizures, a multidisciplinary and multimodality evaluation with MR imaging, nuclear imaging, and subdural localization by electroencephalogram can guide focal resection for treatment with favorable long-term seizure outcomes observed in up to 80% of patients undergoing surgery. Factors associated with recurrent seizures following surgery include operation after 18 years of age, long duration of epilepsy before surgery and the presence of multilobar focal cortical dysplasia.
Disorders of neuronal migration
Lissencephaly
Lissencephaly (literally, smooth brain) is characterized by reduced gyral and sulcal development with patterns of both pachygyria and agyria. There are a variety of gene alterations resulting in lissencephaly, which have classically been grouped into 2 major subtypes: type 1 (classic) and type 2 (cobblestone) lissencephaly or cobblestone malformation.
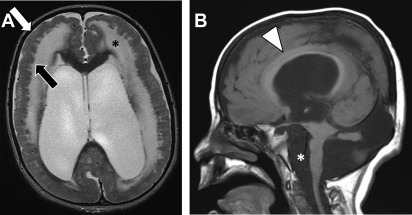
Classic lissencephaly arises from disruption of neuronal migration between the 10th and 14th weeks of gestation. Imaging findings of lissencephaly range from the classic hourglass or figure of 8 shape, reflecting diffusely thickened and abnormally smooth cerebral cortex in its more severe form ( Fig. 3 ), to milder forms of subtle band heterotopias. More severe forms are manifested in infancy with hypotonia, seizures and developmental delay. Milder forms with only subcortical band heterotopia are more commonly observed in heterozygous female carriers of the X-linked DCX gene mutation and may be minimally symptomatic with mild seizures and present later in childhood or in the adult setting.
Cobblestone malformation (once known as type II lissencephaly) results from an overmigration of neurons through the pia and into the subarachnoid space. The best known cause results from a mutation involving the dystroglycan complex, which anchors the neuronal cytoskeleton to the extracellular matrix. This mutation results in a heterogeneous group of disorders, affecting the brain, muscles, and eyes, including Fukuyama muscular dystrophy, muscle-eye-brain disease, and Walker-Warburg syndrome. The imaging appearance ranges from mild pachygyria to a cobblestone multinodular appearance of the cortex, most pronounced anteriorly ( Fig. 4 ).
Clinical presentation typically varies based on the underlying syndrome with varying phenotypes of developmental delay, hypotonia, and visual problems. Muscle-eye-brain disease has the least severe phenotype and may present later in life with mean age of 9 ± 5.5 years (range, 2-19 years) with varying degrees of spasticity and additional imaging findings of periventricular white matter abnormalities, ventriculomegaly, pontocerebellar hypoplasia, and cerebellar cysts.
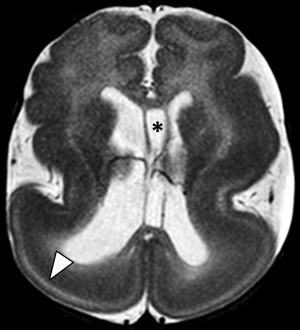
Gray matter heterotopia
Heterotopic gray matter refers to focal collections of gray matter arising from interruptions in normal neuronal migration. They are subdivided into 3 subgroups, which describe their location and include periventricular/subependymal, focal subcortical, and leptomeningeal (double cortex or band heterotopia). The most common presentation is with seizures, with onset and severity depending on the location and degree of heteropia. Patients with subependymal heterotopias have relatively late onset of clinical symptoms manifesting as simple partial seizures and/or developmental delay ( Fig. 5 ). In cases of intractable seizures, which is more common with the leptomeningeal subtype, palliative surgery may be performed.