Congenital entities sharing imaging characteristics with true pathologies occasionally are discovered incidentally in adults. These may occur in the neck, chest, abdomen/pelvis, or musculoskeletal systems. Although these incidental findings share imaging features with true pathologic processes, up-to-date knowledge and assessment with the most appropriate imaging modalities generally allow a distinction between congenital entities that may be safely dismissed and pathologic processes requiring further assessment and treatment. This article reviews several of the most common congenital processes that may present incidentally in adult patients mimicking disease. Emphasis is on findings that can be used to distinguish congenital process from true disease processes.
Key points
- •
Congenital incidental findings are not uncommonly encountered in adults.
- •
Radiographic analysis of the incidental finding frequently provides an inconclusive assessment.
- •
Careful evaluation of ultrasound, computed tomography, and/or MR imaging can help distinguish the incidental congenital process from a true pathologic process.
- •
Radiologists should be familiar with common congenital entities and variants of development that may simulate disease in the adult.
Introduction
Congenital entities and variants of development occasionally are discovered incidentally in adult patients. Many of these incidental findings share similar imaging characteristics with and may mimic true pathologic entities. Persistence of developmental structures, accessory structures from development, and incomplete maturation all may result in an appearance that simulates true pathologic processes, such as infection and neoplasia. Although these congenital incidental findings share similar imaging features with true pathologic processes, up-to-date knowledge and careful assessment with the most appropriate imaging modalities generally can allow a distinction between congenital entities that may be safely dismissed and those pathologic processes requiring further assessment and treatment.
The overarching aim of this article is to review several of the most common congenital processes that may present incidentally in the adult patient mimicking disease. An emphasis is placed on imaging findings that can be used to distinguish the congenital process from disease processes in need of additional evaluation and treatment.
Spectrum of congenital incidental findings
Neck
Ectopic thymus
The thymus begins development during weeks 4 to 6 of gestation, arising from the third branchial pouch. By the end of the eighth to ninth weeks, the primordial thymus descends caudally into the anterior mediastinum and fuses in the midline. Ectopic thymus may be found anywhere along the path of descent from the cervical region to the mediastinum owing to failure or incomplete descent, sequestration, or persistence of a remnant and failure of involution within the thymopharyngeal duct. ,
Thymic tissue is instrumental in development of B cells and T cells and is disproportionally larger in infants, with a gradual involution and replacement by fat with maturation. Cervical ectopic tissue may present as a painless lump or mass in childhood or be discovered incidentally in childhood, adolescence, or adulthood. Ectopic tissue may be positioned laterally or midline in the neck, simulating many other pathologic processes, including lymphadenopathy and primary neck neoplasia ( Fig. 1 ). Ectopic thymic tissue also may be located in the thyroid and is found in approximately 1% of the pediatric and adult population, potentially mimicking malignant thyroid nodules, especially those with microcalcification ( Fig. 2 ).
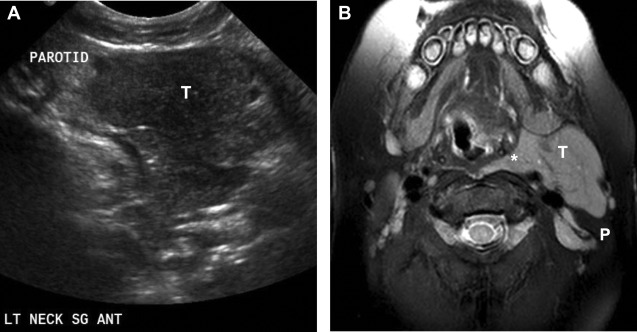
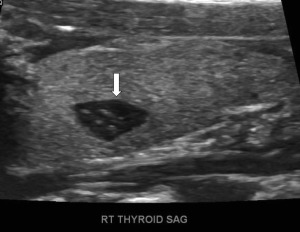
The key to making a diagnosis of ectopic cervical thymus, both within and outside the thyroid, is to recognize the potential for its presence and the characteristic imaging appearance. Normal thyroid tissue has multiple linear hyperechoic septa and discrete homogeneously distributed hyperechoic foci, resulting in a speckled appearance on ultrasound. , On MR imaging, ectopic thymic tissue is homogenous, isointense, or slightly hyperintense to skeletal muscle on T1-weighted images and hyperintense on T2-weighted images and typically has angulated margins that mold to adjacent structures rather than displacing them. In adolescents and adults, the thymic tissue matures, showing progressive involution and an increasingly fatty appearance (after orthotopic, normal thymus maturation), resulting in an increase in echogenicity on ultrasound. Observance of the typical appearance on imaging may obviate biopsy or removal of a significant amount of thymic tissue, which may have detrimental effects on immune system development in younger patients.
Branchial cleft cyst
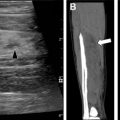
The branchial apparatus develops between the fourth and seventh weeks of gestation and forms the precursors of the ear, blood vessels, bones, cartilage, and mucosal lining of the face, neck, and pharynx. Branchial apparatus anomalies result from incomplete obliteration of the associated cleft or pouch during development and are the second most common congenital neck lesion, behind thyroglossal duct cysts.
Second branchial cleft anomalies are the most common of all of the branchial apparatus anomalies to develop. , Cysts are the most likely anomaly to be seen. Second branchial cleft anomalies are encountered lateral to the midline, along the developmental tract, extending from the tonsillar fossa inferior-laterally between the internal and external carotid arteries to the level of the skin anterior to the sternocleidomastoid (SCM) muscle.
On imaging, the second branchial cleft cysts are seen most commonly anterior to the SCM muscle, resulting in posterior displacement of the SCM, medial displacement of the carotid sheath structures, and anterior displacement of the submandibular gland ( Fig. 3 ). Ultrasound typically shows a well-circumscribed round or oval hypoechoic or anechoic cyst with posterior acoustic enhancement. Occasionally, the internal fluid may be complex and the wall thickened in the setting of infection. Computed tomography (CT) and MR imaging may better elucidate the precise location of the cyst, with attenuation and internal signal characteristics reflecting the complexity of the fluid.
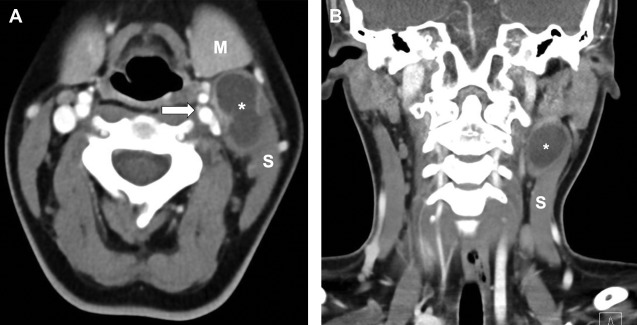
Although many second branchial cleft cysts are diagnosed in the pediatric population, branchial cleft cysts that are small and/or asymptomatic may remain undetected into adulthood. The branchial cleft cyst may mimic other pathologies within the neck, including necrotic lymph nodes and primary head/neck malignancies. One key to differentiation is recognition of their characteristic anatomic positioning, although surgical resection may be required for definitive diagnosis in questionable cases.
Chest
Lung agenesis
Lung agenesis is a rare congenital anomaly. Embryonic development of the lung begins early in gestation, with the development of 2 lung buds that grow to form the left and right mainstem bronchi during the embryonic phase. With pulmonary agenesis, there is complete absence of all normal pulmonary structures on the affected side. The cause of pulmonary agenesis is not definitively known, although it may be related to abnormal blood flow in the dorsal aortic arch during the fourth week of gestation, mechanical or genetic causes, or teratogenic effects. , Approximately 50% of patients with lung agenesis have concomitant congenital anomalies involving the cardiovascular, central nervous, gastrointestinal, and genitourinary systems. , Those with unilateral lung agenesis, however, may reach adulthood and remain asymptomatic. ,
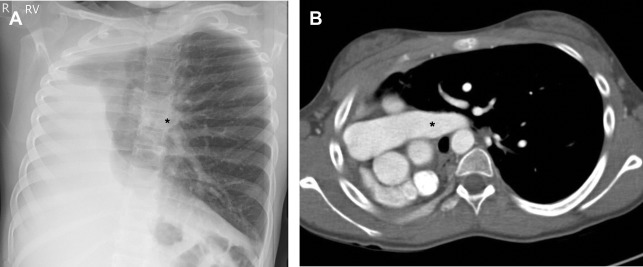
Unilateral lung agenesis results in mediastinal shift toward the hemithorax with absent lung and compensatory hypertrophy and hyperexpansion of the contralateral lung, which herniates across midline. Chest radiography typically shows opacification of the affected hemithorax and ipsilateral mediastinal shift that can mimic complete lung atelectasis and those findings seen postpneumonectomy ( Fig. 4 ). The differential diagnosis for patients with unilateral lung atelectasis is broad but includes obstructing bronchial entities, such as primary lung cancer in adults. CT can help distinguish between an acquired disease process and congenital absence of the lung, revealing the absence of the lung parenchyma, bronchus, and ipsilateral pulmonary artery.
Pulmonary arteriovenous malformation
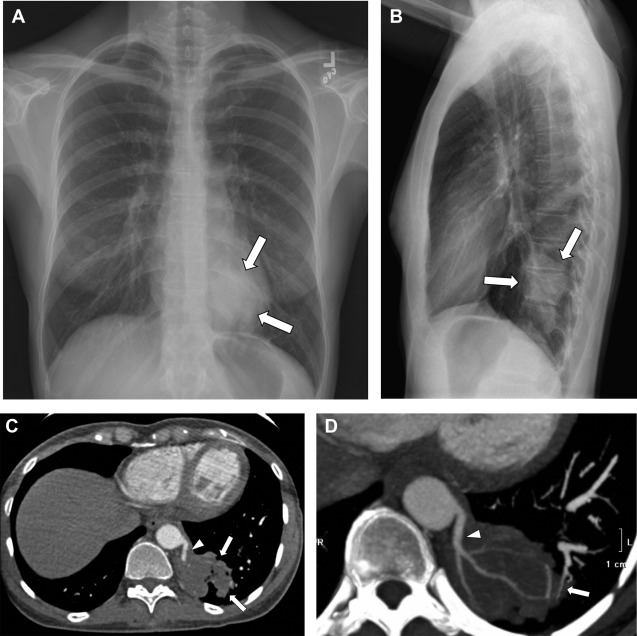
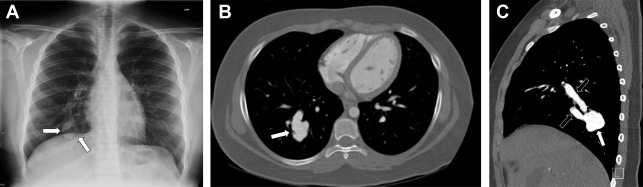
A pulmonary arteriovenous malformation (PAVM) is an abnormal communication between the pulmonary artery and pulmonary vein, bypassing the normal pulmonary capillary network. PAVMs are relatively uncommon in the general population, although they occur more commonly in patients with hereditary hemorrhagic telangiectasia. , PAVMs usually are discovered incidentally in asymptomatic patients, but when large or multiple may result in right-to-left shunting and embolic phenomena.
On chest radiography, PAVMs appear as pulmonary nodules ( Fig. 5 ). When the feeding vessels are large enough to be radiographically conspicuous, they help establish a diagnosis. Smaller lesions, however, may have inconspicuous vasculature, resulting in a pulmonary nodule that has a broad differential diagnosis, including other vascular etiologies, such as pulmonary artery aneurysms, infectious entities, and neoplastic entities, including primary lung cancer—especially in adult patients. CT angiography may be necessary to help distinguish the incidentally discovered asymptomatic PAVM from other entities requiring further investigation and treatment. On CT, PAVMs most commonly appear as well-defined peripheral nodules that may be lobulated, into which a feeding pulmonary artery and at least 1 draining pulmonary vein can be identified (see Fig. 5 ). ,
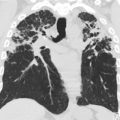
Bronchogenic cyst
Bronchogenic cysts arise from defective foregut budding during early lung development. They may arise in the paratracheal, hilar, or most commonly the subcarinal regions. , More rarely, bronchogenic cysts can be found within the lung parenchyma or in the cervical region. , When the bronchogenic cysts are intrapulmonary, they usually are solitary with thin walls and are seen most commonly in the lower lobes. Affected patients generally present within the first 4 decades of life, although approximately half of patients are asymptomatic with the bronchogenic cyst detected incidentally on an imaging study performed for alternate indications.
Bronchogenic cysts generally appear as a round or oval-shaped opacity on radiographs. CT provides better characterization, especially of internal fluid attenuation, which may have density similar to that of water or soft tissue. , On MR imaging, those bronchogenic cysts with simple internal fluid exhibit high signal intensity on T2-weighted images and low signal intensity on T1-weighted images. Those bronchogenic cysts with internal fluid that is not simple typically display variable signal intensity on T1-weighted and T2-weighted images, reflecting the internal composition of the fluid. The cysts do not have central enhancement on postcontrast MR images.
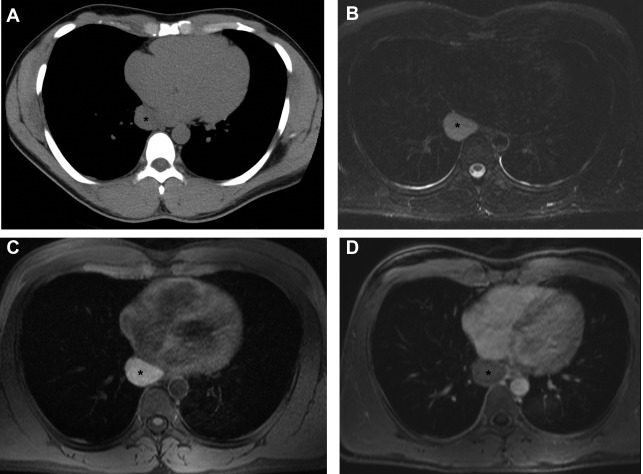
When found incidentally, bronchogenic cysts may mimic other pathologies found in the mediastinum or hilum. Specifically, a bronchogenic cyst identified on CT with low internal attenuation may mimic a necrotic lymph node from an infectious or neoplastic source. , When solid-appearing on CT, bronchogenic cysts may mimic solid lymph nodes or neoplasms. MR imaging may provide additional helpful information on the characteristics of the fluid and, when contrast is given, reveal the uniformly enhancing thin wall of the cyst and lack of central enhancement ( Fig. 6 ). In distinction, solid masses and lymph nodes typically show central enhancement and those masses with central necrosis are less likely to have a uniformly thin wall rather than a thick or irregular wall.
Pulmonary sequestration
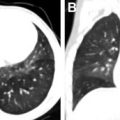
Pulmonary sequestrations are the second most common congenital pulmonary anomaly (behind congenital pulmonary airway malformations). They are characterized by a portion of dysplastic lung not in connection with the tracheobronchial tree that have a systemic arterial supply, generally from the thoracic or abdominal aorta, and either pulmonary or systemic venous drainage. , , Two types of pulmonary sequestrations have been described based on venous drainage pattern and pleural investment: the intralobar types do not have their own pleural investment and generally drain via the pulmonary venous system and the extralobar types have their own pleural investment and drain via the systemic venous system. Intralobar pulmonary sequestrations are more common than extralobar pulmonary sequestrations and although some investigators suggest intralobar pulmonary sequestrations may be acquired after birth, at least some of these pulmonary sequestrations are congenital because they are identified on prenatal imaging. , ,
Although extralobar pulmonary sequestrations are diagnosed in the prenatal-neonatal period, intralobar pulmonary sequestrations are found more commonly incidentally in childhood or adulthood at chest radiography. , , Pulmonary sequestrations tend to appear as an area of increased opacity, usually in the lower lobes, that may mimic other pathologic processes, such as pneumonia. With recurrent infection, the pulmonary sequestration may develop intralesional necrosis, generating a more cystic appearance, occasionally with air-fluid levels. The necrotic appearance can further mimic cavitary variants of pneumonia or a neoplastic process. CT with intravenous contrast generally is needed for diagnosis. The use of intravenous contrast and angiographic technique can highlight the systemic feeding artery(s) and the draining veins, confirming the diagnosis of pulmonary sequestration ( Fig. 7 ).