Hematopoietic stem cell transplantation is an intravenous transfusion of pluripotent stem cells to repopulate the marrow and restore immunocompetence. However, before transplantation, the patient undergoes a conditioning regimen to eradicate the underlying disease, subsequently resulting in an immunocompromised state. Serious and some life-threatening complications involving any organ can occur. Currently, with advances in hematopoietic stem cell transplantation techniques and posttransplant management, more pediatric patients are now living longer and into their adulthood. The goal of this review article is to discuss the common neurologic, pulmonary, and abdominal complications associated with hematopoietic stem cell transplantation with emphasis on their imaging characteristics.
Key points
- •
Hematopoietic stem cell transplantation is transfusion of pluripotent stem cells harvested from the bone marrow, peripheral blood, or fetal cord blood into a recipient to repopulate the marrow and restore hematologic and immunologic competence.
- •
Various complications are common and may be classified according to time frames following transplantation where the patient’s immune status is unique.
- •
There is predominance of specific infectious pathogens and disease patterns in each phase.
- •
Imaging appearances of cerebral infections differ from that in immunocompetent hosts but cerebrovascular complications are similar to those seen in the general population.
- •
Imaging appearances are nonspecific and require microbiologic and/or histopathologic correlation.
Introduction
Hematopoietic stem cell transplantation (HSCT) is an intravenous transfusion of pluripotent stem cells to repopulate the marrow and restore immunocompetence. However, before transplantation, the patient undergoes a conditioning regimen to eradicate the underlying disease, subsequently resulting in an immunocompromised state. Serious and some life-threatening complications involving any organ can occur. Currently, with advances in HSCT techniques and posttransplant management, more and more pediatric patients are now living longer and into their adulthood.
The goal of this review article is to discuss the common neurologic, pulmonary, and abdominal complications associated with HSCT with emphasis on their imaging characteristics as pediatric patients transition into their adulthood.
Background information: hematopoietic stem cell transplantation
HSCT is a procedure where pluripotent stem cells are intravenously transfused into a recipient to repopulate the marrow and restore hematologic and immunologic competence. Stem cells can be harvested from the bone marrow, peripheral blood, or fetal cord blood. It is said to be autologous when the cells come from the same patient, syngenic when from an identical twin , and allogenic when from another donor. ,
Common clinical indications for HSCT for both children and adults include hematologic malignancies such as leukemia, lymphoma, myelodysplastic syndrome, and multiple myeloma; nonmalignant hematologic disorders such as thalassemia and anemia, specifically sickle cell and aplastic types; solid malignancies; and immunodeficiencies. ,
Before transfusion, the patient undergoes a conditioning regimen that involves total-body irradiation and/or high-dose chemotherapy to eradicate the underlying disease and to suppress the immune system, decreasing the risk of rejection. However, this results in an immunocompromised state.
Various serious and life-threatening complications can occur even with advances of medications including immunosuppressants and antibiotic agents. Complications may be classified according to time frames following transplantation where there is a unique immune status of the patient. , ,
First is the preengraftment phase , which includes the time of pretransplant conditioning and up to 30 days posttransplantation. This is characterized by severe bone marrow suppression with pancytopenia particularly neutropenia, disruption of mucosal barrier, cellular and humoral immunodeficiency, and functional asplenia. ,
The second phase begins after successful engraftment of the stem cells. This is the early posttransplantation or early postengraftment phase , which is about 30 to 100 days. In this phase, there is recovery of the neutrophils and mucosal injury, but the delay in the lymphocyte counts results in persistent cellular and humoral deficiency, as well as functional asplenia. , The first and second phases may also be called the peri-engraftment period , which is up to 100 days posttransplantation.
After 100 days, the late posttransplantation or late postengraftment phase ensues. There is relative immune reconstitution with recovery of the lymphocyte counts, but depending on clinical circumstances, the cellular and humoral immune function will recover after a year. ,
Posttransplant complications may occur in any organ system, but this review article only focuses on the more common neurologic, pulmonary, and abdominal complications with their imaging characteristics that are encountered in clinical practice.
Complications associated with hematopoietic stem cell transplantation
Neurologic Complications
Neurologic complications occur in 11% to 59% of patients undergoing HSCT. , Complications could be infectious and noninfectious in nature.
Infectious complications
Preengraftment phase (up to 30 days)
The most common neurologic complication is infection. Imaging appearance of cerebral infections differs from that in immunocompetent hosts. The classic imaging findings of infection may be absent due to impaired inflammatory response. There is less or no rim enhancement, mass effect, or edema around the cerebral lesions. , , ,
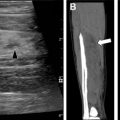
The most common central nervous system (CNS) infections (30%–50%) are caused by invasive Aspergillus . , , , Invasive aspergillosis initially develops in the paranasal sinuses and lungs. Extension into the brain occurs by 2 main possible mechanisms. One is via hematogenous spread from the lungs, which causes either infectious vasculopathy leading to multiple randomly distributed infarctions or hemorrhages in 25% of cases or extension into surrounding tissue resulting in cerebritis or abscesses. Secondly, it can also spread contiguously through the paranasal sinuses, where it can cause brain abscesses, cavernous sinus thrombosis, or carotid artery pseudoaneurysms. , ,
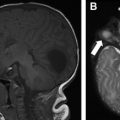
More than 90% of cerebral abscesses following HSCT are caused by Aspergillus . Lesions are usually multiple and located in the basal ganglia, thalamus, and subcortical white matter ( Fig. 1 ). , ,

Computed tomography (CT) typically demonstrates multiple low-attenuation foci, with minimal mass effect and negligible contrast enhancement. MR imaging can detect more lesions than CT when evaluating intracranial extension of the disease. Increased signal on T1-weighted images may be due to hemorrhage or the manganese content of the fungus. On T2-weighted images, lesions may have peripheral low signal intensity, better appreciated on gradient recall echo sequence or susceptibility-weighted images, likely from surrounding hemorrhage. The lesions can also have a peripheral ring of restricted diffusion corresponding to infarction from angioinvasion. Ringlike enhancement may be absent. , , ,
Early postengraftment period (days 31–100)
Viral infections
The herpes group of viruses is the most likely cause of neurologic complications, although routine prophylaxis has greatly reduced the incidence of encephalitis due to herpes simplex virus, varicella zoster virus (VZV), and cytomegalovirus (CMV). , ,
Human herpes virus 6 (HHV-6) encephalitis after allogenic HSCT is a serious complication occurring in up to 78% of patients, usually during either early or late posttransplant phases. It causes posttransplant acute limbic encephalitis (PALE). , , PALE manifests with anterograde amnesia, changes in mental state, headache, fever, and drowsiness. MR imaging typically shows symmetric, bilateral T2-weighted and fluid-attenuated inversion recovery (FLAIR) hyperintense lesions in the limbic system particularly at the medial temporal lobe, hippocampus, and amygdala. , , ,
Toxoplasma infection
Parasitic CNS infections due to Toxoplasma gondii , are almost always fatal, with a frequency of 0.3% to 13%. , Clinical symptoms are nonspecific, usually presenting with fever, seizure, headache, or altered mental status. MR imaging shows multiple lesions in the basal ganglia, , , posterior fossa, or at the gray-white matter junction. On T2-weighted images, lesions can be hyperintense due to necrotizing encephalitis or isointense from organizing abscess.
Imaging appearance of toxoplasmosis after HSCT is different from those in the human immunodeficiency virus (HIV)-infected population. Lesions are initially hemorrhagic following HSCT, whereas in HIV infection, hemorrhages are only seen after initiating antitoxoplasma therapy. Edema and contrast enhancement may also be absent unlike in HIV infection. The lesions may have peripheral diffusion restriction due to hemorrhage within the walls but not within the center as seen in pyogenic abscesses.
Bacterial infections
Gram-positive bacterial infections also frequently occur in this period. , , Methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis as well as Listeria monocytogenes may cause severe meningitis and ventriculitis. ,
Meningitis presents with hyperintense extra-axial spaces with leptomeningeal enhancement on the postcontrast FLAIR images. Contrast-enhanced FLAIR sequence is more sensitive and specific than contrast-enhanced T1. For pyogenic ventriculitis, the typical MR imaging findings are hydrocephalus, abnormal ependymal enhancement, and intraventricular debris with diffusion restriction, which is highly sensitive. ,
Late postengraftment period (more than 100 days)
CNS aspergillosis is common and tends to present in a median of 131 days after HSCT. , The most important risk factor is prolonged neutropenia. , It may manifest as meningitis, abscess formation, or vascular invasion, leading to thrombosis, infarction, hemorrhage, or mycotic aneurysms. Imaging reveals isointense to low signal intensity lesions on T2-weighted images in the cerebral hemispheres, basal ganglia, thalami, corpus callosum, cerebellum, or brainstem. Diffusion-weighted imaging may show restriction suggesting early ischemic changes caused by fungal embolism.
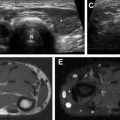
Because of antiviral prophylaxis, VZV infection emerges later and continues beyond the first 6 months. This may cause vasculitis, which may lead to thrombosis, resulting in cerebral infarction and rarely hemorrhage. MR imaging demonstrates multiple infarctions in the cortices, gray-white matter junctions, deep central gray and white matter, and brainstem. Although the aforementioned are typical features, it may also present with nonspecific manifestations and can mimic HHV-6 encephalitis ( Fig. 2 ). Conventional or MR angiography may reveal stenosis or occlusion of the major arteries in the circle of Willis.

Noninfectious central nervous system complications
Peri-engraftment period (up to 100 days)
Cerebrovascular complications
Cerebrovascular complications most often manifest in the preengraftment or in the early postengraftment period, occurring in 3.8% to 8.8% of patients following HSCT. ,
The most common hemorrhagic complication during bone marrow depletion is a subdural hematoma. It is usually associated with underlying acute leukemia and persistent thrombocytopenia. , , ,
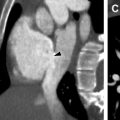
Intraparenchymal hemorrhages ( Fig. 3 ) are more common in allogenic HSCT particularly when accompanied by severe graft-versus-host disease (GVHD). This is probably related to microvascular endothelial injury or vasculitis. , , ,

Infarctions may also occur in this phase and may be the result of using dimethylsulfoxide in autologous HSCT, fungal emboli, or with prolonged neutropenia. According to a retrospective study by Coplin and colleagues, nearly one-third of strokes were caused by Aspergillus fumigatus , Staphylococcus endocarditis (5.6%), and noninfectious endocarditis (2.8%). ,
Subdural hematomas, infarctions, and intraparenchymal hemorrhages are common complications, and imaging characteristics are similar to those seen in the general population.
Posterior reversible encephalopathy syndrome
Posterior reversible encephalopathy syndrome (PRES), also known as posterior reversible leukoencephalopathy syndrome, is the most commonly reported neurologic complication after pediatric allogenic HSCT. The incidence is 1.1% to 20%. , It is most common during the early posttransplant period but can occur in any stage. , PRES is also a known complication of pretransplant steroids and calcineurin inhibitors such as tacrolimus and cyclosporine A, used as GVHD prophylaxis. , , , , , , PRES manifests with rapid onset of seizures primarily, but hypertension, cortical blindness, headache, vomiting, and altered mental status may follow. , , The pathophysiology remains unclear, but theories entail a breakdown of the blood-brain barrier leading to vasogenic edema (6.8).
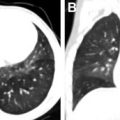
CT is the first-line imaging modality, mainly used to exclude hemorrhages, , , but MR imaging is the current imaging modality of choice. The characteristic imaging appearance consists of fairly symmetric ( Fig. 4 A), bilateral vasogenic edema without diffusion restriction ( Fig. 4 B) in the cortical-subcortical gray and white matter of the parietooccipital lobes in 50% to 99%. , , , Atypical PRES may involve the frontal and temporal lobes, cortical watershed zones, basal ganglia, brainstem, and cerebellum. , , , , Other findings may include contrast enhancement (20%); restricted diffusion (11%–26%); and hemorrhages (5%–19%) including petechial hemorrhage (<5 mm), subarachnoid hemorrhage, and intraparenchymal hematoma. ,

Most of the patients with PRES resolve completely with no detectable residual abnormalities ( Fig. 4 C), but residual gliosis may happen when infarcts or hemorrhages do occur. , , , , Complete clinical recovery usually precedes neuroimaging resolution. ,
Wernicke encephalopathy
Metabolic encephalopathies are also common but occur less frequently than other CNS complications. They usually occur in the preengraftment or early postengraftment period. , Prolonged fasting, hyperemesis, and prolonged total parenteral nutrition may result in Wernicke encephalopathy. , , The most frequent manifestation is a change in mental status. MR imaging may reveal symmetric, hyperintense T2 and FLAIR signal in the mammillary bodies, medial thalami, around the aqueduct of Sylvius and in the tectal plate. Contrast enhancement is most common in the mammillary bodies. , ,
Late posttransplantation stage (more than 100 days)
In the late phase after HSCT, malignancies can develop in either of the following forms: CNS relapse and therapy-induced neoplasms.
Relapse of the underlying CNS disease can also occur, especially with leukemia or lymphoma. The clinical presentation can mimic the late neurologic sequelae of HSCT.
Patients who undergo treatment of hematopoietic malignant disease, especially with high doses of total-body irradiation, are at high risk for developing a solid malignant tumor within 10 years or more. CNS tumors such as glioblastoma, astrocytoma, lymphoma, and meningioma can occur. , The risk of malignant brain tumors is 4.3 times higher in allogenic HSCT. , ,
Thoracic Complications
Pulmonary complications occur because of the toxicity of the pretransplantation medications and the effects of pancytopenia, with a frequency of 40% to 60%. However, noninfectious pulmonary complications are more common due to the increasing use of prophylactic antibiotics. The imaging modality of choice following transplantation is CT.
Preengraftment phase (up to 30 days)
Infectious complications
Fungal infections account for 25% to 50% of all pneumonias in allogenic transplant recipients. The most frequent fungal pathogen is Aspergillus , followed by Mucor and Candida . ,
Aspergillus pneumonia can occur at any time following transplantation. It happens mostly but not exclusively in allogenic recipients, with a prevalence of 10% to 15%. Two forms have been described, namely angioinvasive aspergillosis, which is more common, and airway-invasive (tracheobronchial) aspergillosis. ,
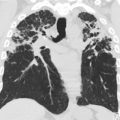
Angioinvasive aspergillosis manifests as multiple pulmonary nodules or masses, parenchymal opacification, or a combination of these, with upper lobe predominance. On CT, the nodules may be surrounded by ground-glass haziness (“halo” sign) ( Fig. 5 ), which is a common, but not specific, sign because it may also be seen in other infectious causes including Candida , Pseudomonas , CMV, and Actinomycosis infections. The halo represents hemorrhage around an area of infarction and necrosis secondary to invasion and blockage of the venules by the fungus. This may be seen within 2 weeks of infection. The “air crescent” sign may also be seen, which is a crescent-shaped lucency within a nodule or area of consolidation. This is a good prognostic sign because it reflects neutrophil recovery, thus observed later in the disease process. , Detection of galactomannan, a cell wall antigen, in the blood or bronchoalveolar lavage fluid is highly sensitive and specific (>90%) for invasive pulmonary aspergillosis.