Brain tumors can develop in the prenatal and neonatal time periods. Neuroimaging studies are crucial for the early detection of prenatal and neonatal brain tumors. Imaging allows for characterization of morphology, as well as the detection of hydrocephalus, local invasion, and distant spread. The imaging features of the more common neonatal brain tumors, including teratomas, choroid plexus tumors, ATRTs, and neoplasm mimics are described.
Key points
- •
Neonatal brain tumors are rare and account for fewer than 2% of all pediatric brain tumors.
- •
Most brain tumors that present within the neonatal period (first 4 weeks after delivery) develop prenatally and may be diagnosed in utero with obstetric ultrasound imaging or fetal MR imaging.
- •
Teratoma, a subtype of germ cell tumors, is the most common brain tumor in neonates. On imaging, teratomas typically are well-defined, large, heterogeneous masses with contrast-enhancing solid portions, nonenhancing cystic portions, fatty tissue, and mineralization.
- •
Choroid plexus tumors are the second most common brain tumors found in neonates, commonly found in the lateral ventricle and often presenting with hydrocephalus. On MR imaging, they demonstrate a typical frondlike appearance, and avid contrast enhancement.
- •
Atypical teratoid/rhabdoid tumor (ATRT) is a primitive neoplasm that is a World Health Organization grade IV and is markedly aggressive with a universally dismal prognosis. The imaging appearance of ATRT is very similar to that of other embryonal tumors; however, ATRT often demonstrates a dramatically rapid growth pattern not seen with other tumors.
Introduction
The neonatal period is defined as the period of first 4 weeks after delivery. Brain tumors that present within the neonatal period are discussed in this article. Most of these develop prenatally and may be diagnosed in utero with obstetric ultrasound imaging or fetal MR imaging. Neonatal brain tumors are rare and represent 0.5% to 1.9% of all pediatric brain tumors. Several of the previously published series on neonatal brain tumors relied on data collected before the wide availability of neuroimaging with computed tomography (CT) or MR. The availability of high-resolution imaging during the fetal and neonatal periods makes the early diagnosis of these tumors possible, often at a subclinical stage. Advanced neuroimaging techniques have improved our understanding of the histologic and anatomic distribution and behavior of these tumors. With this improved understanding of neonatal brain tumors. it is likely that the previously published prevalence may not be a true reflection of the incidence of neonatal brain tumors.
Wakai and colleagues presented their categorization of congenital brain tumors to include brain tumor cases in infants presenting up to the first 2 months of life. Clearly tumors presenting at birth are congenital brain tumors. Thereafter, the confidence regarding the congenital or neonatal origin of brain tumors decreases with the increase in time between birth and presentation. More slowly growing brain tumors that develop during the neonatal period may not become apparent until the child is a year or older. Hence, several neonatal brain tumors that grow slowly may not be included in this category. In this article, we review imaging features of the brain tumors when they present in the neonatal age group.
The most recent update (2016) in the World Health Organization (WHO) classification of central nervous system (CNS) tumors has significantly changed the classification of a number of tumor families. This 2016 update has, for the first time, included molecular parameters into the diagnostic scheme. The most common neonatal brain tumor is teratoma, a subtype of germ cell tumors, followed by choroid plexus tumors. Another large group of neonatal brain tumors, the embryonal tumors, include embryonal tumors with multilayered rosettes (formerly known as primitive neuroectodermal tumor [PNET]), medulloblastomas, and atypical teratoid/rhabdoid tumors (ATRTs). ATRTs are a unique group of embryonal tumors that tend to occur in young children and neonates. The astrocytic tumors and tumors of neuronal and mixed neuronal-glial neuronal tumors, such as desmoplastic infantile astrocytomas (DIA) and gangliogliomas (DIG), are also typically found in the neonates. Meningeal tumors and hematopoietic tumors also can rarely present in the neonatal period. The more commonly occurring brain tumors in the neonate are presented in Box 1 .
- •
Germ cell tumors: teratoma (mature and immature)
- •
Choroid plexus tumors (papilloma and carcinoma)
- •
Embryonal tumors
- ○
Embryonal tumors with multilayered rosettes (formerly primitive neuroectodermal tumor)
- ○
Atypical teratoid/rhabdoid tumor
- ○
Medulloblastoma
- ○
- •
Astrocytic tumors
- •
Neuronal and mixed neuronal-glial tumors:
- ○
Desmoplastic infantile tumors (astrocytomas and gangliogliomas)
- ○
Introduction
The neonatal period is defined as the period of first 4 weeks after delivery. Brain tumors that present within the neonatal period are discussed in this article. Most of these develop prenatally and may be diagnosed in utero with obstetric ultrasound imaging or fetal MR imaging. Neonatal brain tumors are rare and represent 0.5% to 1.9% of all pediatric brain tumors. Several of the previously published series on neonatal brain tumors relied on data collected before the wide availability of neuroimaging with computed tomography (CT) or MR. The availability of high-resolution imaging during the fetal and neonatal periods makes the early diagnosis of these tumors possible, often at a subclinical stage. Advanced neuroimaging techniques have improved our understanding of the histologic and anatomic distribution and behavior of these tumors. With this improved understanding of neonatal brain tumors. it is likely that the previously published prevalence may not be a true reflection of the incidence of neonatal brain tumors.
Wakai and colleagues presented their categorization of congenital brain tumors to include brain tumor cases in infants presenting up to the first 2 months of life. Clearly tumors presenting at birth are congenital brain tumors. Thereafter, the confidence regarding the congenital or neonatal origin of brain tumors decreases with the increase in time between birth and presentation. More slowly growing brain tumors that develop during the neonatal period may not become apparent until the child is a year or older. Hence, several neonatal brain tumors that grow slowly may not be included in this category. In this article, we review imaging features of the brain tumors when they present in the neonatal age group.
The most recent update (2016) in the World Health Organization (WHO) classification of central nervous system (CNS) tumors has significantly changed the classification of a number of tumor families. This 2016 update has, for the first time, included molecular parameters into the diagnostic scheme. The most common neonatal brain tumor is teratoma, a subtype of germ cell tumors, followed by choroid plexus tumors. Another large group of neonatal brain tumors, the embryonal tumors, include embryonal tumors with multilayered rosettes (formerly known as primitive neuroectodermal tumor [PNET]), medulloblastomas, and atypical teratoid/rhabdoid tumors (ATRTs). ATRTs are a unique group of embryonal tumors that tend to occur in young children and neonates. The astrocytic tumors and tumors of neuronal and mixed neuronal-glial neuronal tumors, such as desmoplastic infantile astrocytomas (DIA) and gangliogliomas (DIG), are also typically found in the neonates. Meningeal tumors and hematopoietic tumors also can rarely present in the neonatal period. The more commonly occurring brain tumors in the neonate are presented in Box 1 .
- •
Germ cell tumors: teratoma (mature and immature)
- •
Choroid plexus tumors (papilloma and carcinoma)
- •
Embryonal tumors
- ○
Embryonal tumors with multilayered rosettes (formerly primitive neuroectodermal tumor)
- ○
Atypical teratoid/rhabdoid tumor
- ○
Medulloblastoma
- ○
- •
Astrocytic tumors
- •
Neuronal and mixed neuronal-glial tumors:
- ○
Desmoplastic infantile tumors (astrocytomas and gangliogliomas)
- ○
Clinical presentation
Clinical presentation of neonatal brain tumors varies depending on the type, size, and location of the tumor. Most common presenting signs are increasing head circumference, vomiting, and lethargy. A bulging fontanelle and setting-sun sign may also frequently be noted. Other presenting signs may include seizures, focal motor deficits, hemiparesis, cranial nerve palsy, and nystagmus. Cases diagnosed prenatally may have delivery complications including prolonged labor, fetal distress, and failure of labor progression, typically related to large head size.
Imaging
Head ultrasound and unenhanced brain CT are the most common initial imaging modalities when neonatal brain tumors are suspected. In utero detection of congenital brain tumors is most often incidental, with screening or routine obstetric ultrasound, and better characterized with the increasing use of fetal MR imaging. The neonatal brain can be assessed with head ultrasound via the sonographic window created by the open fontanelles. Although detection of a mass is possible with ultrasound, cross-sectional imaging is required for further evaluation. CT imaging is quick and usually can be performed with swaddling of the neonate, without requiring sedation. Calcification and acute hemorrhage are easily detected with CT; however, CT scanning exposes the neonate to ionizing radiation. MR imaging with its multiplanar imaging capability, high signal-to-noise ratio, and superior ability to characterize tumors and their impact on surrounding structures, does not involve ionizing radiation. However, MR imaging may require sedation or, in some cases, general anesthesia. Advanced imaging sequences, such as perfusion, diffusion tensor imaging, and susceptibility weighted imaging can be extremely helpful in better characterizing the tumor types and their relationship(s) to eloquent brain regions. Volumetric acquisitions facilitate intraoperative imaging guidance, and can be performed with CT and MR imaging.
Regardless of the tumor type, the dominant imaging appearance of neonatal brain tumors is that of a large, heterogeneous-appearing mass, usually with hydrocephalus and macrocephaly.
Germ cell tumors
Teratomas, a subtype of germ cell tumors are the most common brain tumor in neonates, accounting for approximately 33% to 50% of cases. Intracranial is the third most common location, after sacrococcygeal and cervico-facial. Teratomas arise from multipotent cells and, as a result, usually produce tissues that represent an admixture of 2 or more of the embryologic layers of ectoderm, mesoderm, and endoderm. A supratentorial location is seen in approximately in two-thirds of cases, most commonly associated with the structures about the third ventricle; the pineal gland region is the leading site of origin, followed by the suprasellar region. Congenital teratomas also may involve the cerebral hemispheres more extensively.
Mature (benign) teratomas are composed exclusively of well-differentiated, “adult-type” tissue elements. Mitotic activity is low or absent. The more common ectodermal components encountered in such tumors include skin, teeth, neural elements, and choroid plexus. Mesodermal representatives include cartilage, bone, fat, smooth and striated muscle, and choroid plexus (noting its dural embryologic origin). Cysts lined by respiratory or enteric epithelium account for endodermal elements and have been reported to contain thyroid, pancreatic, or hepatic tissue.
Immature (malignant) teratomas contain incompletely differentiated components, resembling fetal tissue. Presence of any incompletely differentiated areas mandate classification of the lesion as immature, even a small component in an otherwise well-differentiated tumor. Some immature teratomas contain malignant cells of conventional somatic type. The most common of these are rhabdomyosarcoma and undifferentiated sarcoma. Production of alpha-fetoprotein by the glandular epithelium in an immature teratoma may result in elevated levels of this biomarker in the serum and cerebrospinal fluid (CSF). Increased serum carcinoembryonic antigen (CEA) also may be commonly found in patients with teratoma.
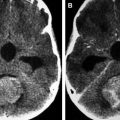
On imaging, mature teratomas typically are well-defined, T1 and T2 heterogeneous masses with contrast-enhancing solid portions, nonenhancing cystic portions, fatty tissue, and mineralization. On CT they commonly appear as mixed density masses with any fatty components appearing hypodense; CT also has a high sensitivity for mineralization. This association of fat, mineralization, and heterogeneous solid and cystic tissue is highly suggestive of a teratoma. On MR images, mineralization may be more challenging to detect than on CT. However, with the use of T2 gradient echo and susceptibility weighted imaging, it is possible to reliably identify mineralization in teratomas on MR imaging. MR fat-suppression techniques and CT may be useful in differentiating fat from hemorrhage. Contrast enhancement is usually heterogeneous and is limited to the solid areas and along the walls of the cystic spaces ( Fig. 1 ). Immature teratomas may show less well-defined margins and larger solid to cystic portion ratios. They are typically large and usually lacking in calcification and fat ( Fig. 2 ). Immature teratomas are often larger than mature teratomas at presentation and infiltrate surrounding structures. Peritumoral edema is often observed around immature teratomas; this is usually absent with mature teratomas. CSF dissemination is common with immature teratomas. Imaging characteristics may suggest the type of teratoma; however, classification requires histologic examination. Intracranial teratomas are increasingly being diagnosed antenatally ( Figs. 3–5 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree