6
Breast Brachytherapy
Simona F. Shaitelman, Chirag Shah, Leonard H. Kim, Frank A. Vicini, Douglas W. Arthur, and Atif J. Khan
Breast-conserving therapy (BCT), consisting of segmental mastectomy followed by whole-breast irradiation (WBI), has become widely accepted as an alternative to mastectomy as a treatment for women with earlystage breast cancer. Accelerated partial-breast irradiation (APBI) is a shorter, alternative radiation technique for select patients with favorable-risk, early-stage breast cancer. In this chapter, we review different APBI modalities delivered using brachytherapy techniques. Notably, this chapter does not discuss or review literature and techniques for external beam–based APBI.
BCT, comprised of breast-conserving surgery and adjuvant breast radiotherapy, is comparable to total mastectomy with regard to the rates of local-regional control and overall survival, while enabling patients to retain the breast (1,2). Based on the initial trials conducted in the 1970s and 1980s, BCT has typically involved partial mastectomy and some degree of lymph node surgery, followed by WBI over the course of 5 weeks, with or without a “boost” treatment to the tumor bed. With more than 20 years of follow-up and the cumulative experience of tens of thousands of patients, WBI results in good long-term cosmetic results and is associated with low rates of treatment-related morbidity. A meta-analysis published by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has demonstrated that omitting radiotherapy from breast-conserving surgery can compromise local control and result in a modest detriment in overall survival (3). For these reasons, thought leaders have cautioned that altering conventional radiation treatment to target only part of the breast must be carefully scrutinized, because a decrease in local control may translate into a decline in overall survival when patients are followed for a long period of time. However, a closer look at the most recent update of the EBCTCG meta-analysis focusing only on intact breast trials quite clearly demonstrates that compromises in local control among the most favorable-risk subset of patients does not lead to compromises in breast-cancer specific and all-cause mortality. As such, modest compromises in local control in select populations may be acceptable, provided effective salvage strategies are available and survival is not compromised.
RATIONALE
APBI is a treatment approach in which only the area of the breast where the tumor was initially located plus a margin is targeted with radiation; treatment can be delivered over 1 to 15 days because of the smaller treatment volume. In contrast to WBI, which typically encompasses most of the breast parenchyma, chest wall musculature, ribs, and approximately 60% of the level I and II lymph nodes, APBI focuses radiation on a 1 to 2 cm margin of tissue surrounding the lumpectomy cavity (Figures 6.1A and B).
The rationale for irradiating only part of the breast is supported by data from three randomized trials that recorded the location of breast failures after lumpectomy with or without radiotherapy (RT). These trials quite consistently demonstrate that the majority of breast cancer recurrences following lumpectomy alone occur adjacent to the lumpectomy cavity (1,4,5). Indeed, in these studies, the incidence of treatment failures elsewhere in the breast, far from the initial tumor bed, occurred at a similar rate in patients who received WBI as in those who did not, implying that these elsewhere failures were not affected by WBI. Irradiating less of the breast can reduce the dose to uninvolved breast tissue, lungs, ribs, chest wall musculature, and the heart, which may in turn reduce the risk of late complications and improve cosmesis (6,7).
A separate rationale for APBI comes from data examining residual disease and patterns of extension in pathological specimens. However, these data are mixed and need to be viewed with some caution. A frequently cited paper from Holland et al reported using whole-mount mastectomy specimens to examine extension of disease beyond a simulated lumpectomy (8). The original data reported high rates of occult disease existing at distances of 2 cm and beyond the original tumor site. However, the applicability of these data to modern patients with small, mammographically detected in situ and invasive lesions treated with current surgical techniques is questionable. More recent pathological data suggest that disease extension beyond the index lesion is uncommon (9–13). However, it is important to note that these data do indicate an approximately 10% risk of microscopic disease beyond a 1 cm perimeter from the index lesion.
Compared to a 5 to 6 week WBI treatment, it has been argued that the shorter course of APBI will improve patient satisfaction and overall quality of life, potentially minimizing the psychological and physical strain associated with radiation treatment (14–16). For those patients who live far from a radiation oncology facility or who suffer from multiple medical comorbidities, a shorter treatment might improve compliance with completing the prescribed course of radiation and/or minimize the election of mastectomy over BCT (17–21). Recent analyses by Shah and Lanni demonstrated that brachytherapy-based APBI reduces the overall financial cost of treatment compared to WBI (22,23), refuting earlier work that used outdated assumptions of reimbursement (24,25).

Figure 6.1 (A and B) Axial and sagittal images of the targeted areas in APBI. Contoured in red is the seroma/lumpectomy cavity, the green area is the clinical target volume, and the blue color outlines the whole-breast contour. APBI, accelerated partial-breast irradiation.
PATIENT SELECTION
Beginning in 2007, four separate oncologic societies have published guidelines to assist physicians in selecting patients for APBI offered off protocol (Table 6.1). The American Society for Radiation Oncology (ASTRO) organized a task force that published the most detailed set of guidelines regarding suitability to receive APBI off protocol. This group recommended that women be classified as “suitable” for APBI treatment if they are aged 60 years or older and have invasive ductal (or other favorable histology) carcinoma less than or equal to 2 cm in size; estrogen receptor positive; unicentric and unifocal; lymph node negative; with negative margins (greater than or equal to 2 mm); and with no extensive intraductal component (EIC), lymphovascular space invasion (LVSI), or neoadjuvant therapy (26). The Groupe Européen de Curiethérapie and European Society for Therapeutic Radiology and Oncology (GEC-ESTRO), the American Society of Breast Surgeons, and the American Brachytherapy Society have all published their own sets of guidelines (27–29). These four sets of guidelines were based on older published studies and the inclusion criteria used therein, coupled with the judgment of experienced breast radiation oncologists.
It is worth noting that none of the criteria defining those patients considered to be ill suited to receive APBI were based on published reports of worse outcomes in those patient subsets. Indeed, most of the factors listed in the guidelines are related to general risk factors for the recurrence of breast cancer in the setting of BCT. To our knowledge, none of the criteria listed is known to directly correlate with a patient’s risk of having a higher rate of recurrence if she receives radiation to the whole breast compared to the lumpectomy cavity only. We posit that additional pathologic data mapping the extent of disease could help better determine suitability to receive APBI.
TREATMENT TECHNIQUES
Various treatment modalities have been used to deliver APBI, incorporating different techniques and targeted volumes of breast tissue, including brachytherapy, intraoperative techniques (X-rays, electrons), and external radiation with photons and/or protons. Each modality offers advantages and disadvantages, depending on patient anatomy and preference, as well as the resources and expertise available at a particular radiation oncology facility. Ideally, each patient is assessed for the technique that will best meet her individual needs.
Multicatheter Interstitial Brachytherapy
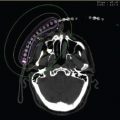
Multicatheter interstitial brachytherapy (MIB) is the APBI technique with the longest follow-up and the most mature data. MIB involves the percutaneous placement of approximately 10 to 20 catheters in the breast tissue surrounding the lumpectomy cavity (Figure 6.2). This procedure can be performed under local anesthesia and is usually done after final pathology results from the initial surgery have returned. Low (LDR) or high dose rate (HDR) radioactive sources are temporarily loaded into the catheters in a way that allows target dose delivery to the tumor bed and a margin of 1 to 2 cm. Because of the properties of the radioactive sources used, MIB allows for highly conformal treatment, with near complete sparing of surrounding normal tissues (7).
The Radiation Therapy Oncology Group (RTOG) conducted a phase II, multi-institutional study, RTOG 95–17, which included 99 patients with early-stage breast cancer treated with LDR or HDR brachytherapy to deliver APBI. In this study, the 10-year rate of ipsilateral breast tumor recurrence (IBTR) was 6.2%, and half the recurrences were outside the area of the treatment field (30). Investigators at the National Institute of Oncology in Hungary presented their 10-year follow-up of a randomized trial of 258 women randomized to APBI or WBI, with APBI delivered either via MIB HDR brachytherapy or external electron beam irradiation (31). The rates of recurrence were not significantly different between the two arms (5.9% for APBI vs 5.1% for WBI), and patients treated with APBI had higher rates of good-to-excellent cosmesis (81% vs 63%, P = .009).
Table 6.1 Published consensus statement criteria for the delivery of accelerated partial-breast irradiation off protocol

Figure 6.2 (A) Multicatheter interstitial brachytherapy and (B) composite dosimetry. Green outline is the PTV, the white line is the 150% isodose, red line is the 100% isodose, yellow line is the 85% isodose and the dark blue line is the 50% isodose. The breast parenchyma is drawn in pink. PTV, planning target volume. Image courtesy of Dr. H. Kader, MD, FRCPC.
Critics of this technique point to its invasiveness and the associated risk of infection and scarring from multiple entry and exit sites. Among the prospective data published on interstitial brachytherapy used to deliver APBI, the rates of infection range from 0% to 11%, with the majority of studies citing rates less than 5% (31–34). A recent interim report on toxicity from the randomized GEC-ESTRO trial reported by Strnad et al demonstrated lower rates of acute and late skin toxicity with MIB compared to WBI (35). For reasons likely related to current training structures in postgraduate programs and physician preferences in North America, MIB is perceived as technically complex, requiring expertise on the part of both the physician and the physics staff, which is the primary reason it has not gained greater traction. In contrast, there is a high degree of proficiency using MIB in Europe, as evidenced by the successful accrual to the GEC-ESTRO trial.
Single-Entry Intracavitary Brachytherapy Catheters
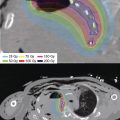
In response to growing clinical interest in APBI and the desire for a simpler insertion technique, single-entry brachytherapy catheters emerged as a way to deliver APBI using brachytherapy. This technique is simpler also for physicists and dosimetrists and less invasive for patients. A catheter is inserted by either the breast surgeon or radiation oncologist through a single percutaneous puncture site in the breast and positioned within the lumpectomy cavity. Most commonly, these insertions happen into a closed cavity, under ultrasound guidance, after the pathology has been reviewed. However, intraoperative placements are possible, but less desirable given the longer time the catheter must remain within the patient while the pathology is reviewed. At the completion of treatment, the catheter is removed with or without local anesthetic in the physician’s office. Unlike with MIB, early, single-lumen versions of these brachytherapy devices were restricted to patients with specific geometric characteristics, that is, nonsuperficial surgical cavities. More recent multiple-lumen devices allow greater flexibility in treatment planning, enabling both better coverage of the target tissue at risk and decreased dose to the nearby structures such as ribs and skin (36,37). These multi-lumen devices are either balloon based with multiple interior lumens or strut based, which directly abut the breast parenchyma (Figures 6.3 and 6.4A and B).
Figure 6.3 Axial image of a treatment plan using a balloon-based brachytherapy catheter. Volume in light blue colorwash is the PTV, the blue line corresponds to the 200% isodose line, red to the 150% isodose line, and green to the 100% isodose line. PTV, planning target volume.

Figure 6.4 (A and B) Orthogonal CT images of a strut adjusted volume implant brachytherapy catheter. Volume in light blue colorwash is the PTV, and the following colors refer to the respective isodoselines listed: white, green, yellow, red, purple, and blue: 200%, 150%, 125%, 100%, 75%, and 50%. PTV, planning target volume.
Since the U.S. Food and Drug Administration (FDA) approved the original single-lumen MammoSite RTS (Hologic) catheter in 2002, the use of brachytherapy is believed to have increased dramatically. A study of Medicare billing claims among women treated with BCT estimated that the use of brachytherapy as a component of oncologic care increased in incidence from less than 1% of cases in 2001 to 10% of cases by 2006 (38). The American Society of Breast Surgeons has enrolled and followed a cohort of 1,440 women treated with the single-lumen MammoSite catheter on a registry trial. This group has reported a 5-year actuarial IBTR rate of 3.8% (39). Of the patients in this study, 90.6% had good-to-excellent cosmesis, 13.0% developed symptomatic seromas, 9.5% developed an infection, and 2.3% developed fat necrosis. These results are comparable to those seen with MIB (40). However, recent claims-based population studies have raised concerns on the toxicities of brachytherapy-based APBI compared to those of WBI. Smith et al conducted a retrospective population-based cohort study of 92,735 women aged 67 years or older with invasive breast cancer diagnosed between 2003 and 2007, treated with BCT, and followed through 2008 (41). The study compared 6,952 women treated with brachytherapy to 85,783 treated with WBI. The 5-year incidence of subsequent mastectomy was slightly higher among women treated with brachytherapy (4.0% vs 2.2%). It remains uncertain whether this difference in mastectomy rates was due to local recurrence (no data were available regarding the rate of local recurrence) or to toxicities that required surgical treatment, or to other unknown reasons (eg, laterality of the mastectomy compared to the initial tumor was unknown); it is also unclear whether this incremental difference negatively offsets the convenience of accelerated treatment or if it has any impact on overall survival. Brachytherapy was also associated with a higher rate of infectious and noninfectious complications, including breast pain, fat necrosis, and rib fracture. A similar study by Presley et al reported on 29,648 Medicare beneficiaries aged 66 to 94 years treated with BCT between 2008 and 2009 (42). There was a significantly higher rate of wound and skin complications among the 15.8% of women who received brachytherapy instead of WBI (adjusted rate of 33.7% vs 16.8%, P < .001), based on billing claims, a rate that far exceeds anything published in the prospective literature. Notably, there was a significant geographic variability in the frequency of treatment delivery with brachytherapy nationally, a finding also demonstrated in a Surveillance, Epidemiology, and End Results (SEER) database study looking at national trends (43). This finding suggests that nonclinical factors may have influenced the implementation of brachytherapy-based APBI. These studies have the benefit of providing information about a broad cross-section of patients treated throughout the United States, with large patient numbers. These studies however, lack information about the clinical and tumor characteristics of the patients to whom treatment was given, making it difficult to ascertain whether treatment was given in a way that would be deemed most appropriate.
Intraoperative Radiation Therapy
Although brachytherapy-based APBI has enjoyed acceptance by way of professional society endorsement for specific patients, the utility and appropriate use of single-fraction radiotherapy as a method of APBI remains contentious and undefined. There are three major causes for concern with current intraoperative radiation therapy (IORT) methods: (a) patients are selected for a treatment strategy without full pathological review of data; (b) dose to the target volume is not consistent with known radiobiology; and (c) the treating physicians are unable to graphically evaluate and monitor doses delivered to the target volume or normal tissues. Recent updates of the two seminal randomized trials of IORT completed in Europe demonstrate results that are inferior to both WBI and fractionated APBI.
The TARGeted Intraoperative radioTherapy (TARGIT) approach employs an intraoperative spherical applicator to deliver a single dose of 20 Gy at the applicator surface with 50 kV X-rays, which results in a dose of 5 Gy at 1 cm. There is concern that the dose delivered by TARGIT is inadequate for the control of microscopic disease, even after correcting for the relative biologic effectiveness of a 50 kV beam (44). Due to the physical constraints imposed by this technology, a higher dose at depth would create an unacceptably high dose at the surface of the applicator and the breast tissue it contacts. A total of 2,232 women were randomized to either fractionated WBI (with or without a boost) or to TARGIT at a dose of 20 Gy at the applicator surface. Patients were grouped into two strata: the prepathology stratum included patients who had their definitive lumpectomy and TARGIT at the same sitting, and the postpathology stratum that consisted of patients who were taken back to the operating room for the TARGIT treatment after final pathology had been reviewed. Notably, 21% of patients in the prepathology stratum required subsequent WBI on account of unfavorable pathological features (45). Patients on the postpathology stratum were selected for TARGIT after pathological review and did not receive WBI. In the most recent results, a total of 3,451 patients were randomized and followed for a median of 2 years and 5 months (46). The 5-year IBTR rate is higher in the TARGIT-treated patients than those treated with WBI, 3.3% versus 1.3%, P = .042. The 5-year rate of IBTR in the postpathology stratum (ie, well-selected patients, none of whom required “remedial” whole-breast RT) was surprisingly high at 5.4% (compared to 1.7% for whole-breast RT).
With the ELIOT approach, a homogeneous dose is delivered to a target volume that is generally consistent with the target volumes treated in the accumulated brachytherapy APBI literature. However, the ELIOT approach does not take final pathology findings into account; no “remedial” treatment is offered to women found to have positive margins or positive lymph nodes. The impact of positive margins on local failure is well documented. Margin status from the primary lumpectomy specimens is not reported in the ELIOT data; however, roughly 25% of patients in both groups were node positive, with approximately 5% having pN1b disease (greater than three positive nodes). The ELIOT results highlight the perils of poor patient selection that may come with IORT. Furthermore, the clinical fat necrosis rate in the ELIOT patients on the randomized trial was much higher (14%) than the 2.3% reported with balloon device-based APBI (40). This is likely because the 21 Gy dose was arrived at using an α/β ratio of 10 for breast cancer, which is now understood to be incorrect (48). While acknowledging the imperfection of linear-quadratic assumptions at doses from 8 to 12 Gy, the ELIOT dose is almost double the 2 Gy equivalent dose of fractionated courses of APBI. Thus, the ELIOT experience is fraught with pessimism secondary to suboptimal tumor control and higher toxicity.
A summary of the published and closed but not yet reported randomized controlled trials comparing brachytherapy-based partial breast irradiation to WBI can be seen in Tables 6.2 and 6.3, respectively.
Noninvasive Brachytherapy Boost Treatment
A noninvasive breast brachytherapy system has been commercially developed with a goal of delivering a precise dose to the tumor bed as a part of the tumor bed boost typically given as part of WBI. In this system, the breast is immobilized between mammographic paddles along two orthogonal axes, following which applicators are used, through which HDR iridium-192 (192Ir) is directed at the tumor bed (49). This technique takes advantage of the use of mammographic images to guide the tumor bed boost, with the goal of more accurately targeting the area at highest risk of recurrence. Preliminary data on the implementation of this technique for the delivery of boost treatment has shown good cosmetic outcomes, comparable to those with external beam-based boost (50). (See the example in Figures 6.5A and B.)
TREATMENT PLANNING
The most commonly used dose scheme for accelerated partial-breast brachytherapy using HDR is 34 Gy in 10 twice-daily fractions over a 1 week period (51–53). This dose fractionation scheme is used for both multicatheter and applicator-based brachytherapy. An alternate schedule used is 40 Gy in eight twice-daily fractions. Similar dose-delivery schemes using LDR, pulsed dose rate (PDR), and HDR are in use and have been reported with successful results (54,55). Efforts at compressing the fractionation to a 2 day treatment course have been reported from the William Beaumont Hospital and a collaborative multi-institutional group (56,57).

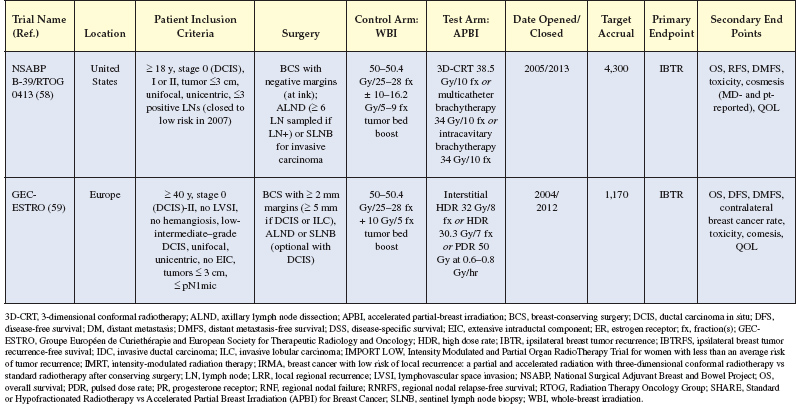
Figure 6.5 (A) The clinic target is localized by mammography and the applicators opposed over the target. (B) A highly conformal dose is delivered in a four-field-like technique with no exit dose to the chest wall, heart, or lung. Images courtesy of Shirin Sioshansi, MD.
Successful treatment planning and dose delivery depends on the appropriate delineation of a treatment target and a thorough quality assurance program. Two-dimensional treatment planning methods should no longer be used. With the availability of CT scan-based three-dimensional (3D) brachytherapy treatment planning, target volumes can be clearly outlined and dosimetric coverage documented.
The treatment target is the lumpectomy cavity plus a 1 to 2 cm margin. It should be recognized that the dimensions of the treatment target are bounded by the limits of breast tissue extension. Equating the target dimensions regardless of treatment method is necessary for standardization of APBI but presents a challenge. Multicatheter brachytherapy has the ability to vary the target volume as desired by the treating physician, by deliberately placing catheters in the area of interest. On the other hand, applicator-based brachytherapy is more dosimetrically restrictive and dependent on the relationship between the final applicator dimensions and lumpectomy cavity characteristics. Although the dose delivered appears to be limited to a nominal treatment distance of 1 cm from the balloon surface, there is debate if the actual treatment distance may be deeper, depending on possible stretching of the circumferential tissue as the balloon is inflated (60). Dickler et al demonstrated that a 1 cm nominal treatment distance from the balloon or applicator surface may represent a greater volume of uncompressed breast tissue (61). However, this contention has not been supported by the work of others. Indeed, at least one report has suggested that the effective treatment volume for balloon brachytherapy may be smaller than that for external beam radiotherapy (62). Strut Adjusted Volume Implant (SAVI) based-brachytherapy more likely and closely mimics interstitial catheters with regard to any possible compression or stretching of adjacent breast parenchyma.
Once the target has been clearly delineated, dosimetric coverage can then be assured. Target delineation carries with it a degree of uncertainty due to operator dependence and the accuracy of the imaging software used. As a result, holding to the ideal 100% target coverage with 100% of the prescription dose may be difficult (63). Relaxing the dose coverage goals would appear appropriate until clinical data suggest otherwise. Although striving for optimal coverage is appropriate, a minimum requirement of at least 90% of the desired dose delivered to at least 90% of the delineated target is fairly standard and is the metric endorsed in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B39 study.
On the basis of these and other reports, the NSABP B39/RTOG 0413 phase III protocol randomizing patients to partial-breast irradiation or WBI requires that implants be designed to minimize both the V150 and V200, while still maintaining adequate planning target volume coverage of at least 90% of the prescribed dose received by 90% of the target. The V150 must be less than or equal to 70 mL for an interstitial HDR implant and less than or equal to 50 mL for a MammoSite brachytherapy. The adopted V200 guidelines are less than or equal to 20 mL for an HDR implant and less than or equal to 10 mL for MammoSite brachytherapy. The SAVI single-entry brachytherapy catheter incorporates a hybrid of the dosimetric parameters used for interstitial and for balloon-based brachytherapy, with a suggested V200 < 20 mL and V150 < 50 mL (69).
A major advantage of HDR therapy over LDR treatment is the ability to alter source dwell times to compensate for suboptimal implant geometry (70). Minor changes in dwell times have been shown to increase the percentage of the target receiving the prescribed dose from 87% to 97%. However, this improvement in coverage resulted in an increase in the mean V150 from 26 cm3 to as much as 70 cm3, which may lead to an increase in toxicity. Consequently, HDR treatment offers some flexibility in dosimetric planning, but the alteration of dwell times cannot fully compensate for poor implant geometry.
With the use of the MammoSite RTS, balloon symmetry, cavity conformance, and skin thickness are important parameters to evaluate to ensure proper use. Deviation in these parameters may affect dosimetric target coverage and the degree of dose homogeneity. The skin separation distance and the related dose to the skin are important parameters that require close monitoring, especially when treating with a single-lumen device. This has a direct impact on the risk of toxicity and long-term cosmesis. Increased acute and late skin toxicity has been reported when the distance from the balloon surface to the skin is less than 7 mm (71). With the advent of multicatheter single-entry applicators and dwell-time optimization, it has been recognized that maximum skin dose should replace applicator-to-skin distance as a plan evaluation metric. The NSABP B39 protocol acknowledges both of these metrics, recommending an applicator-to-skin distance of 7 mm and specifying a surface dmax limit of 145% of prescription. Although the 7 mm applicator-to-skin distance is still supported by toxicity studies for single–lumen balloons, a dmax surface limit of approximately 125% of prescription is now recommended to reduce risk for most skin effects, while other toxicities such as telangiectasia may benefit from an “as low as possible” approach to organs at risk (OAR) dose (72). Indeed, a recent report demonstrated lower rates of telangiectasia when the skin dose was less than 100% of the prescription dose (73). Although the newer multilumen applicators allow optimization, these asymmetric dose distributions require new quality assurance procedures, particularly for the evaluation of applicator rotation (50,74).
Investigations in breast brachytherapy have followed larger trends in the field toward adaptive therapy and more accurate dose calculation. In the realm of adaptive therapy, serial imaging has suggested that the applicator-to-surface distance may decrease over a treatment course. Although dose-toxicity studies based on initial planning doses may implicitly incorporate this effect on average, unusual changes may prompt replanning; for example, the resolution of air pockets surrounding a balloon applicator, which may motivate replanning to adjust target coverage (75,76).
In the area of dose calculation, it has long been recognized that a primary condition behind Task Group (TG)-43 dose calculation, that is, that the dose is calculated in a water-equivalent medium large enough to provide full scatter, is not met in breast brachytherapy. Thus, there have been numerous investigations on deviations from the TG-43 dose, particularly near tissue–air interfaces where full scatter conditions are lacking. Such locations include air pockets near balloon and SAVI applicators as well as critical structures like the skin and chest wall. The effects of high-density media such as radiographic contrast in balloon applicators and bone (particularly for electronic brachytherapy) have also been investigated (77–81). It has also been suggested that changing planning structure definitions, for example, defining the skin organ at risk as a volume rather than a surface (82) or including air pockets into the target structure prior to expansion (83), might have greater anatomic and dosimetric validity. Large differences have been reported under certain conditions, and so such studies have value for accurate dose reporting, especially for intermodality comparison. However, it should be noted that if these dose differences are consistent and systematic relative to TG-43, then traditional dose metrics established by older toxicity studies should still be valid for treatment planning, with little if any difference in expected clinical impact. Different dose metrics may be needed to fully exploit advances in these areas.
As discussed, breast brachytherapy dose is prescribed at the target margin. Dose within the target is higher and reaches a maximum at the applicator. Equivalent uniform dose-type calculations have been reported for balloon and interstitial brachytherapy, characterizing this dose inhomogeneity and its effect on toxicity (84,85), as well as facilitating comparison with external beam radiotherapy (86). One open question in this area is the interaction of brachytherapy dose gradients with likely nonuniform distribution of target cancer cells around the applicator. Studies suggest that islets of microscopic disease are both smaller and less likely to be present with larger distance from a breast primary (87,88). The implications of this phenomenon for different accelerated partial-breast modalities are not yet well understood.
BENEFITS AND RISKS
Quality-of-Life Benefits
Patients receiving brachytherapy-based APBI typically are grateful for being offered the option of a more condensed treatment. Still, there has been a lack of published data obtained from surveying women in order to better understand their feelings regarding the convenience of finishing radiation treatment within 1 week with twice-daily treatments compared with daily treatment over 3 to 6 weeks; thus, it remains difficult to quantify this benefit, which results in physicians relying on anecdotal stories of individual patients they have treated. One recent study found that women treated with brachytherapy-based APBI experienced a faster recovery from treatment-related fatigue and improved restoration of their baseline quality of life compared to those patients receiving WBI (16).
One of the original rationales behind APBI was that it would enable more women to have access to BCT—women for whom it would have been too difficult to undergo a more prolonged course of WBI and who might otherwise have opted to pursue mastectomy for this reason alone. To the best of our knowledge, there has not yet been a study documenting the trends in utilization of BCT compared to mastectomy because APBI has become more broadly used, and we would welcome research toward this end. The population-based studies that have been published have found that brachytherapy-based APBI has been, paradoxically, more prevalent in metropolitan areas or their environs (89). This difference in treatment delivery may be due to both patient preference and available technical resources and expertise in urban areas compared to more rural areas. Thus far, there has been no published documentation on the trends in usage of external beam–based APBI in such locales.
THE COSTS OF APBI
The overall clinical cost of APBI, to both patients and society, has not been fully quantified. A shorter course of external-beam APBI clearly costs less than a protracted course of external beam WBI in terms of the amount billed to the patient, the number of health care resources used, and the financial cost to the patient in terms of time away from work and family (22,24). However, since the inception of single-entry brachytherapy catheters, patterns of reimbursement have changed significantly. With this technique, typically there is a financial charge for the catheter and the placement of the catheter by the surgeon. In addition, this treatment technique is significantly more time intensive to the physician and medical physicist involved in the case, and reimbursement has varied over time, with the amount paid increasing in accordance with the number of lumens treated, a reflection of the greater complexity of the case. A few publications analyzing the cost of APBI compared with WBI have resulted in mixed findings. In general, these studies have shown that external beam–based APBI is significantly less expensive than standard fractionation WBI over 5 to 6 weeks. However, the comparison of brachytherapy-based APBI to WBI with a boost is more complicated, and the costs of these two approaches appear generally comparable; however, such things as intensity-modulated radiation therapy (IMRT) treatment plans for WBI and the brachytherapy catheter itself add additional costs. Indeed, a recently published analysis demonstrated that brachytherapy-based APBI remains a cost-effective option based on accepted metrics of cost-effectiveness such as cost per quality-adjusted life-year (cost/QALY) (23).
Most APBI regimens require twice-daily treatment, with a minimum 6 hour time interval between treatments, a regime for which many patients require time off from work. However, these patients typically finish treatment within 1 week instead of the traditional 6 weeks of standard WBI, which means a less prolonged interruption of normal work and home activities. This difference might be minimized as hypofractionated WBI continues to increase in use, allowing treatment of the whole breast in 3 to 4 weeks.
Brachytherapy-based APBI is more labor intensive from the perspective of the breast surgeon, radiation oncologist, and medical physicist. This treatment requires close communication and synchronization of scheduling between the breast surgeon and the radiation oncologist, which can be challenging in a busy clinical practice. In addition, the Nuclear Regulatory Committee requires the attendance of both a radiation oncologist and a medical physicist at the delivery of each fraction of radiation for brachytherapy and intraoperative treatment, in addition to daily quality assurance by the medical physicist. By our estimates, this adds at least 10 extra hours worked per patient compared with external beam-based treatment.
APBI: THE FUTURE
At least four randomized trials comparing WBI and APBI have been presented thus far, in at least partial form (Table 6.2); the results regarding toxicity and efficacy have been mixed. In the future, we anticipate the publication of results from at least eight randomized controlled trials comparing WBI and APBI, two of which use brachytherapy for APBI (Table 6.3). These trials are being conducted in North America and Europe and in total will include more than 16,000 women. The largest of these trials, the NSABP B39/RTOG 0413 trial, is being conducted in the United States, and recently completed accrual. This trial will include 4,300 women with early-stage breast cancer who are being randomly assigned to receive either WBI (with or without a tumor bed boost) or APBI. In this study, APBI can be delivered via MIB, single-entry brachytherapy catheter, or 3D conformal external beam radiation. While the currently open trials vary in terms of patient inclusion criteria and how APBI is delivered, we expect that they will provide definitive information on the equivalency of APBI to WBI. However, given the differences in the techniques used to deliver APBI in these studies, the findings of one study may not be applicable to the technique used in another, in terms of either tumor control or toxicity. The publication of results from these randomized controlled trials, including over 16,000 women, should better characterize the efficacy and safety of APBI in comparison to WBI.