William R. Reinus (ed.)Clinician’s Guide to Diagnostic Imaging201410.1007/978-1-4614-8769-2_4
© Springer Science+Business Media New York 2014
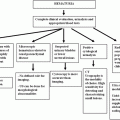
4. Breast Imaging
(1)
Department of Radiology, Temple University Medical School, Philadelphia, PA 19140, USA
Abstract
Breast imaging is a clinical subspecialty within the larger field of radiology. One in eight American women will develop invasive breast cancer during their lifetime. According to the American Cancer Society’s estimates, about 232,340 new cases of invasive breast cancer will be diagnosed in women in 2013, with approximately 40,000 deaths. There are projected to be additional 64,640 new cases of in situ (noninvasive) breast cancer [1]. Breast cancer is the second leading cause of cancer death in women behind only lung cancer. In recent years, increased publicity has made women acutely aware of the risk of developing this disease.
Breast imaging is a clinical subspecialty within the larger field of radiology. One in eight American women will develop invasive breast cancer during their lifetime. According to the American Cancer Society’s estimates, about 232,340 new cases of invasive breast cancer will be diagnosed in women in 2013, with approximately 40,000 deaths. There are projected to be additional 64,640 new cases of in situ (noninvasive) breast cancer [1]. Breast cancer is the second leading cause of cancer death in women behind only lung cancer. In recent years, increased publicity has made women acutely aware of the risk of developing this disease.
History of Breast Imaging
Efforts to use imaging to evaluate breast disease with radiography began shortly after Wilhelm Roentgen discovered X-rays. Despite all the efforts over the years, clinically effective use of mammography was not truly available until the 1960s. Besides radiography, many other modalities aimed at diagnosing breast cancer have been tried. Thermography was used in the 1950s purporting to measure heat emanating from breast tumors due to their neovascularity. This modality did not prove clinically effective, and although recent efforts have been made to reintroduce thermography, its effectiveness has not improved. Although some women still choose thermography because their breasts do not have to be compressed while performing a thermogram, it should not be used as a substitute for mammography [2]. Xeroradiography was introduced in the 1970s. In this technique, a plate coated with selenium rests on a thin layer of aluminum oxide. The X-ray beam passes through the breast and strikes the selenium plate, causing a charge distribution on the plate. Then, as with a paper copier, the image formed on the charged plate is transferred to paper for display. While xeroradiography produced usable images, it required too much radiation, and image storage and reproducibility were significant problems.
By 1960s, mammography was considered clinically safe although the radiation dose to the breast and shielding were not as good as they are today. The radiation dose currently delivered from a two view analogue mammogram has been reduced to approximately 2.37 mGY [3], about the equivalent amount of ambient radiation one receives from the atmosphere in 3 months. At the time the reproducibility, safety, and relative ease of performance also helped to make mammography a more viable option.
While mammograms became more available in the 1960s, it took time for its utility to be understood and utilized by the public. It was not until the mid-1980s that screening programs started to be promoted and until the 1990s that breast cancer awareness exploded with a marked increase in fundraising and cancer research. From the early 1960s when only 10–15 % of women took advantage of mammography, the number of women having yearly screening increased to a high of approximately 75 % in the early 2000s. As a result of strict adherence to a program of yearly mammographic screening after age 40, breast cancer deaths in women between 40 and 50 years of age who are screened have decreased by as much as 26–29 % [4].
The original technique of screen film mammography (analogue) while extremely useful does have limitations. Penetrating through breast parenchyma, particularly in dense breasts, is problematic. Dense parenchyma makes it more difficult to see masses and discern faint calcifications. Compressing the breasts during mammography helps improve penetration and makes lesions more conspicuous. Still, analogue mammography was not ideal.
The need for better techniques led to development of full field digital mammography (FFDM) which is now state of the art. Digital mammography has slightly worse spatial resolution than analogue mammography, but has much improved contrast resolution, allowing for better visualization of calcifications. With a digital display, post-processed magnification is easy and allows a much closer look at all areas of the breast. In addition, digital images permit computer-aided detection (CAD) techniques where a computer marks areas in the breast that it perceives as a mass or calcifications for closer examination after initial interpretation. The radiation dose with digital mammography has decreased to an average of approximately 1.86 mGy per study [3]. Digitized images are transported easily on disc, an aid in our highly mobile society to radiologists who need to compare all old studies to the current one to make an accurate assessment of the current study.
The FDA approved tomosynthesis, the next innovation in breast imaging, for clinical use in 2011. This is essentially a digital tomogram of the breast allowing review of the mammogram in 1 mm “slices.” Studies are being conducted currently to assess value of one-view tomosynthesis vs. two-view FFDM vs. one-view tomosynthesis in combination with FFDM. One recent study showed that one-view tomosynthesis had better sensitivity and negative predictive value than FFDM in patients with fatty or very dense breasts [5].
In the process of attempting continually to improve and refine abilities to diagnose breast cancer earlier, an increased ability to diagnose benign conditions confidently was a significant by-product. Today, breast imaging is a vital component of overall management of breast health and disease, helping to diagnose benign as well as malignant conditions.
Screening Recommendations
In 2009, the United States Preventive Services Task Force recommended that women begin having screening mammograms at age 50, with follow-up mammograms every 2 years thereafter. It also stated that breast self-examination should not be performed [5]. Although an authoritative body, the conclusions of this study were spurious, the result of a flawed meta-analysis of 20 years of retrospective data. As a result, the medical community has rejected the recommendations of this study.
Current breast screening guidelines and recommendations from the American College of Radiology and American Cancer Society are for women at average risk for developing breast cancer to have a baseline mammogram at age 40 with annual follow-up mammograms [1, 6]. Furthermore, these guidelines recommend that breast self-examination begin at age 20. No age has been specified at which to stop obtaining yearly mammograms. It had been suggested that yearly mammographic screening stop at age 85, but today longer life expectancy has made that recommendation obsolete.
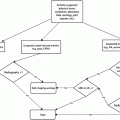
There are specific indications to begin breast screening in women before age 40 [7]:
Carriers of BRCA gene.
Untested first-degree relatives (mother, sister, daughter) of known BRCA carrier.
First-degree relative of a woman diagnosed before menopause with breast cancer. Screening should begin 10 years before the age at which the relative was diagnosed or between the ages of 25 and 30, whichever is later.
Women who have received mantle radiation for Hodgkin’s disease.
Women with any previous biopsy showing atypical hyperplasia of any type (ductal, lobular, lobular carcinoma in situ).
Additional considerations that may prompt earlier commencement of breast screening:
Family history of breast and epithelial ovarian cancer.
Breast cancer in a male family member.
At least two family members on the same side diagnosed with breast cancer.
The Radiologists’ Role
Many women sent for mammograms are very anxious. A commonly held misconception is that having a mammogram is painful, and many women need encouragement to complete the procedure. The need for a breast biopsy magnifies a woman’s anxiety.
Performing interventional procedures is a significant part of the specialty. These include stereotactic biopsy using mammograms as a guide, ultrasound-guided breast biopsies, MRI-guided biopsies, fine needle aspirations (FNAs), cyst and abscess drainage, needle localization of breast lesions as guide to the breast surgeon and occasional galactograms. This means that the radiologist works in close association with the patient’s surgeon and is an active member of the clinical team. The radiologist should meet with the breast surgeon and the pathologist on a regular basis for “concordance conference.” The breast images, pathologic slides, and clinical results of all patients who have had biopsies are reviewed. If pathological results are not concordant with expected results based on imaging and clinical picture, rebiopsy (preferably by a different modality) is recommended.
Breast Imaging Tools
Mammography is the gold standard of breast cancer diagnosis. Many other modalities play ancillary roles in the diagnosis of breast disease. These include ultrasound and magnetic resonance imaging (MRI), neither of which uses ionizing radiation, molecular breast imaging (MBI, BSGI, scintimammography), positron emission mammography (PEM), and PET scanning. With some exceptions, these modalities are rarely used as primary screening tools.
Mammography
Mammography is the primary test used for breast screening and diagnosis. Because of differences in the appearance of breast tissue from woman to woman and even side to side in each woman, there is no “normal” mammogram. Every woman’s baseline mammogram is her normal, and all subsequent mammograms must be compared to her baseline and subsequent mammograms. Any significant changes from baseline or previous mammogram must be evaluated further.
Mammograms, whether analogue or digital (as with all radiographs), can only display five radiographic densities. From least dense to most dense these are air, fat, soft tissue including fluid, bone/calcium, and metal. Normal breast parenchyma is soft tissue density with abundant interlobular fat. Unfortunately, breast tumors, benign breast lesions, e.g., fibroadenomas, breast cysts, hematomas, and mesenchymal lesions (hamartoma, angiomyolipoma, phyloides tumors), and abscesses are all basically soft tissue density and so similar in density to normal breast tissue. Subtle differences in the density as well as perceived morphology and significant asymmetries with remaining breast tissue allow masses to be visualized, some seen better than others. Masses usually require at least one additional modality for further characterization, most frequently ultrasound. This helps provide a more detailed analysis and ultimately either a diagnosis or a decision to biopsy.
Mammograms are done either for screening or for a diagnostic evaluation. Screening mammograms consist of two views of each breast. Diagnostic mammograms are reserved for women with known breast-related complaints or with suspected lesions not fully evaluated on the initial mammogram. Both examinations begin with an interview by the technologist about the patient’s personal breast history.
Screening mammograms are read later by the radiologist after which an official report is generated with recommendations for next necessary study.
Diagnostic mammograms are reviewed by the radiologist while the patient is present in the mammography suite and as many additional views as required are obtained to evaluate the patient’s problem. If the patient has a palpable lesion, typically an ultrasound is performed on the same day.
Radiologists miss at least 10 % of breast cancers on mammography, particularly in women with dense breasts [8]. This means that all women with palpable lesions should have an ultrasound regardless of mammography findings.
Men who complain of swelling in one or both breasts also may require mammography, although it is usually better to begin evaluation with ultrasound. Most often male breast swelling is caused by gynecomastia, but breast cancer does occur in males and has the same mammographic findings as seen in female breast cancer.
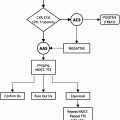
Mammography reporting is standardized according to the Breast Imaging and Data Reporting System (BIRADS) system. This system was developed in an attempt to have uniform reporting throughout the country. It makes recommendations for additional evaluation and so also serves the purpose of guiding the ordering clinician in the patient’s workup. There are seven BIRADS categories [9]:
0.
Further study needed (additional views, ultrasound, MRI).
1.
Normal study. Recommend yearly follow-up mammogram.
2.
Benign finding. Recommend yearly follow-up mammogram.
3.
Probably benign finding. Recommend short-term (6 months) follow-up.
4.
Malignancy suspected. Biopsy recommended.
(May be subcategorized from A to C, according to increasing likelihood of malignancy.)
5.
Malignancy strongly suspected (95 % certainty). Biopsy recommended.
(Also may have A, B, and C subcategories.)