Breast Imaging
Karen K. Lindfors
Huong T. Le-Petross
Breast imaging has two purposes. The first purpose is to screen asymptomatic women for early breast cancer. The second purpose is to evaluate breast abnormalities in symptomatic patients or patients with indeterminate screening mammograms. Screening is accomplished with standard two-view mammography, but diagnostic evaluation often requires the additional use of special mammographic views, breast US, MR, and interventional procedures.
Screening for Breast Cancer
Breast cancer survival is influenced by the size of the tumor and the lymph node status at the time of diagnosis. Small tumors with negative axillary lymph nodes have survival rates well above 90%. Such cancers are detected far more often with screening mammography than with physical examination. It follows that screening mammography should lower mortality from breast cancer. Several randomized controlled trials have proven the efficacy of this technique.
In 1963 the Health Insurance Plan of New York (HIP) invited 31,000 women aged 40 to 64 years to participate in four annual screenings for breast cancer by mammography and physical examination. This study group was compared with a control group of women who received routine medical care. Nine years after beginning the study there was a 29% reduction in breast cancer mortality in the group receiving annual screening (1).
Other trials of mammographic screening were begun in the late 1970s and early 1980s. Four of these were carried out in Sweden and were similar in design. They were population based, meaning that all women living within a specific geographical area who were within the age range under study were included in the trial. Breast cancer mortality was compared between women invited to screening and those not invited (controls). When the data from all centers were combined, the reduction in breast cancer mortality among women aged 40 to 74 years was 24% in the group invited to mammographic screening (2).
The actual benefit of screening mammography for women of all ages is likely to exceed that which has been demonstrated by the randomized clinical trials. Breast cancer mortality data on all women invited for screening, regardless of whether they actually underwent mammography, were used in calculating the reduction of mortality attributable to screening. Compliance rates for obtaining mammography among trial invitees ranged from 61% to 89%. The technology used for mammography has improved greatly since the time that the trials began, resulting in earlier detection of breast cancer (3). Recent evaluations of the impact of mammographic screening in the community setting (service screening) have shown breast cancer mortality reductions of up to 50% among screened women; however, it is difficult to determine the contribution of screening relative to that of improvements in therapy in lowering the death rate from breast cancer (4,5).
Screening Guidelines
Data from the randomized, controlled trials of mammographic screening as well as information from large community-based screening programs were used to formulate the American Cancer Society (ACS) guidelines for breast cancer screening in average risk women, which are shown in Table 20.1 (6). Both
clinical examination and mammography are essential components of a screening program because all cancers are not seen mammographically. False-negative mammograms occur in 9% to 16% of palpable breast cancers.
clinical examination and mammography are essential components of a screening program because all cancers are not seen mammographically. False-negative mammograms occur in 9% to 16% of palpable breast cancers.
Table 20.1 American Cancer Society Guidelines for Breast Cancer Screening in Average-Risk Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
There is controversy over the age at which mammographic screening should begin and also the frequency of such screening. In late 2009, the U.S. Preventive Services Task Force (USPSTF) withdrew its support for mammographic screening for women in their forties and recommended that women ages 50 to 74 years be screened biennially (7). The USPSTF concluded that the benefit gained from screening was not high enough to offset the downsides of screening (false-positive results, anxiety, and possible overdiagnosis and overtreatment). They chose to use a 15% reduction in mortality in their meta-analysis even though mortality reductions of up to 44% have been reported with screening in this age group. The National Cancer Institute advises that women at average risk for breast cancer and age 40 years and older should undergo screening mammography every 1 to 2 years (8). Observational studies have shown that women aged 40 to 49 years were more likely to have late-stage cancers diagnosed if they were screened at 2-year intervals when compared with a 1-year screening interval (9). Other studies of cancers that occur between screens have shown that a greater proportion of breast cancers grow faster in younger women than in older women (10,11,12). It is for this reason that the ACS has recommended annual mammographic screening for women at age 40 and older, yet the chance of being diagnosed with breast cancer between the ages of 40 and 49 is 1 in 66 women or 2%, and the chance of dying from breast cancer is 0.3%. Although it is clear both that mammographic screening can reduce breast cancer mortality for women in their forties and that annual mammographic screening is more effective in reducing breast cancer deaths for women in this age group; economic considerations may favor modifications in screening strategies.
For postmenopausal women, there is some question regarding the additional benefit gained by annual screening since studies have shown that there is no increase in late-stage cancers diagnosed if screening is done every 2 years instead of annually (9). The incidence of breast cancer does increase with age. The age at which mammographic screening should cease is not specified in the ACS or NCI guidelines. There are no data on breast cancer mortality reduction for women who are screened beyond age 74. For elderly women, general health status and quality of life should be considered when deciding whether to undergo mammography. Experts suggest that mammographic screening should stop when life expectancy is less than 5 to 7 years or when abnormal results of screening would not be acted on because of age or comorbid conditions (13).
Women potentially at high risk for development of breast cancer should seek expert advice regarding the age at which screening should begin, the periodicity of mammography, and the possible addition of other screening modalities. A risk assessment should be performed. Factors known to increase a woman’s risk include the following: (1) A personal history of breast or ovarian cancer. (2) Laboratory evidence that the woman is a carrier of the BRCA1 or BRCA2 genetic mutation. These mutations confer an estimated risk of up to 80% for development of breast cancer by age 70. (3) Having a mother, sister, or daughter with breast cancer. (4) Atypical ductal hyperplasia (ADH) or lobular neoplasia diagnosed on a previous breast biopsy. (5) A history of chest irradiation received between the ages of 10 and 30 years. Women who are at high risk (lifetime risk for breast cancer of greater than 20%) should undergo annual screening MR in addition to mammographic screening (14). Screening with US can be considered in high-risk women who cannot undergo MR screening.
When adopting a screening policy, the physician must remember that all women are at risk for developing breast cancer. The ACS estimates that one woman in every eight will develop the disease during her lifetime. The majority of women who contract breast cancer will not have histories that place them at higher risk.
Screening Outcomes
What are the expected outcomes in a group of 1000 asymptomatic women undergoing bilateral screening mammography for the first time? Approximately 80 of these women will be recalled for additional studies. These may include magnification or other special mammographic views and US. Biopsy will be recommended in about 16 of these women, and cancer will be found in about 6 of them. With subsequent screenings of the same women, the numbers of cancers found will decrease and the positive predictive value, or percentage of women undergoing biopsy who actually have cancer, should increase.
The goal of screening asymptomatic women is to find breast cancer in its earliest stages when survival is greatest. In a well-established screening program, over 50% of cancers will be minimal; minimal cancers are defined as those that are noninvasive or invasive, but less than 1 cm in size with negative nodes. Over 80% of breast cancer discovered by screening mammography should be node negative (15,16).
Optimal effectiveness of a breast cancer screening program requires the use of physical examination in addition to mammographic screening. About 9% to 16% of cancers are not visualized mammographically; such cancers are discovered on physical examination. The minimum size of breast cancers that can be felt on physical examination averages between 1.5 and 2 cm.
False-negative mammograms can occur for a variety of reasons. The palpable abnormality may not be included on a film. Dense breast parenchyma may obscure visualization of a mass. The imaging technique may be suboptimal for visualization of an abnormality. The particular tumor type may not be visible mammographically or there may be observer error in the interpretation of the mammogram. It must be emphasized that a negative mammogram should not deter further diagnostic evaluation of a clinically palpable mass.
Some breast cancers will arise in the interval between screening examinations. The number of such cancers will depend on
the frequency of screening. Interval cancers tend to be more advanced at diagnosis when compared with those diagnosed at screening (11); they may be biologically more aggressive. Additionally, a previous negative mammogram or the knowledge that screening will be performed regularly may be a disincentive for patients to seek immediate medical care for a breast mass found in the interval between screens. Physicians must stress that any breast mass requires immediate attention, regardless of whether the patient has had a recent negative mammogram.
the frequency of screening. Interval cancers tend to be more advanced at diagnosis when compared with those diagnosed at screening (11); they may be biologically more aggressive. Additionally, a previous negative mammogram or the knowledge that screening will be performed regularly may be a disincentive for patients to seek immediate medical care for a breast mass found in the interval between screens. Physicians must stress that any breast mass requires immediate attention, regardless of whether the patient has had a recent negative mammogram.
Radiation Risk
An increased susceptibility to breast cancer has been documented among women exposed to high doses of radiation (1 to 20 Gy). The survivors of the atomic bomb explosions in Japan, patients undergoing radiation therapy, and sanatoria patients undergoing multiple chest fluoroscopies for monitoring of tuberculosis therapy are all groups having an increased incidence of breast cancer. Such data raised questions about the risk incurred from the low doses of radiation received during screening mammography (approximately 2 mGy per view).
A controlled study of the effects of low doses of radiation such as those received during mammography would require large numbers of women in both the study and control groups. Close to 100 million patients in each group would be required in order to provide statistically significant data. Clearly, this would not be practical or possible. As such, estimates or risk have been hypothesized by extrapolation from data obtained at higher doses using a linear dose-response model.
Follow-up data from the Japanese atomic bomb survivors have shown progressively decreasing radiation risk with increased age at exposure. Women exposed in their youth and teens suffered the highest increase in risk. No increased risk was demonstrable for women aged 40 years or older at exposure. Studies of the other populations sustaining significant breast radiation exposure have also supported a diminished risk with advancing age at exposure. Estimated lifetime risk of breast cancer death from a single mammogram in the age group from 40 to 49 years is approximately 2 in 1 million. In women aged 50 to 59 years, this risk is reduced to less than 1 in 1 million; progressive reductions in risk are seen at older ages (17).
These theoretical risks should be weighed against the risk of dying from spontaneous breast cancer, which would be approximately 700 per million in women aged 40 to 49 years and 1000 per million in women aged 50 to 59 years. This risk increases steadily with advancing age.
The Use of Other Imaging Modalities for Breast Cancer Screening
Mammography is the only imaging modality that has been proven to reduce breast cancer mortality when used to screen asymptomatic women. MR is being used for breast cancer screening in conjunction with mammography in high-risk women. Other modalities are under investigation for their potential use in screening.
Although the impact of screening with MR on breast cancer mortality remains unknown, prospective studies in high-risk women have shown significantly higher sensitivities for MR screening in addition to mammography (86% to 100%) compared to mammography alone (25% to 60%) (18). MR does, however, have a much higher false-positive rate than mammography and leads to more negative biopsies. It is important to emphasize that MR cannot replace mammography as a screening modality; it must be used as a supplemental method of screening in high-risk women. The addition of MR adds considerable cost to a breast cancer screening program.
Several single institution studies have shown that whole breast screening US can detect small nonpalpable invasive cancers not seen mammographically. Prevalence rates for cancers seen only on sonography are approximately 3/1000, but positive predictive values for biopsies based on US alone are approximately half of those for biopsies of lesions discovered on mammography (19). A multi-institution trial of screening breast US as an adjunct to mammography in high-risk women reported an incremental cancer detection rate of 4.2/1000 women screened with both modalities as compared to mammography alone; however, false positives were also increased substantially when US was used (20).
Studies comparing mammography, breast US, and breast MR for screening in high-risk women have shown that supplemental screening with US adds no benefit to screening with mammography and breast MR. US screening may, however, be useful in high-risk women in whom MR is contraindicated or cannot be tolerated. In addition to high false-positive rates, there are challenges to the incorporation of US as a screening modality. US is highly dependent on the operator and on the equipment and technique used for scanning. It is also a time consuming, labor intensive examination, which should be performed by a radiologist trained in the technique. Automated scanners are under investigation to address these issues, but at present there are concerns about these devices.
Other imaging technologies, such as PET, scintimammography, single photon emission tomography, tomosynthesis, and dedicated breast CT, are also being explored for use in breast cancer detection and diagnosis. Mammography continues to be the single best test for early detection of breast cancer; however, it is likely that in the coming years a more individualized approach based on risk and other factors will be used in breast cancer screening. Newer modalities will be incorporated when appropriate, likely as adjuncts to mammography.
Evaluation of the Symptomatic Patient
Bilateral mammography should be the first imaging study performed in patients older than 30 years who present with breast masses that are suspicious for carcinoma. The mass should be indicated by placing a radiopaque marker over the site. This will assist the radiologist in a targeted mammographic evaluation of this area and will also ensure that the palpable abnormality corresponds to the mammographic abnormality, if one is visualized. Such correlation is important in assuring that the surgical biopsy of a palpable abnormality will encompass the mammographically suspicious area.
The primary reason for performing mammography in a patient with a suspicious palpable mass is to assess the affected breast for multifocal disease and the contralateral breast for suspicious abnormalities that should be biopsied concurrently. Mammography may also be helpful in definitively diagnosing the palpable abnormality as benign, thus avoiding biopsy.
Mammography should be performed before any intervention. A hematoma, resulting from percutaneous fine needle aspiration biopsy, can look similar to a small carcinoma. When such procedures have been performed prior to mammography, it is best to perform a follow-up mammogram 4 to 6 weeks later.
If mammography is negative in a patient with a clinically evident mass and dense breasts, US is often suggested as a subsequent imaging study. US can determine whether the mass represents a simple cyst. Simple cysts are virtually never malignant and do not require aspiration unless the patient has pain
related to the cyst. US cannot provide a specific diagnosis for a solid or complex mass.
related to the cyst. US cannot provide a specific diagnosis for a solid or complex mass.
Alternatively, definitive diagnosis of a palpable mass can usually be made by performing a fine needle aspiration of the mass with a 22-gauge needle. When a simple cyst is present, the aspiration is both diagnostic and therapeutic, as all of the fluid can be withdrawn. In solid or complex masses, a cytologic examination of the cells removed at aspiration will yield the diagnosis.
In younger patients who present with breast masses, mammography must be used more judiciously. This more cautious approach is based on data from the atomic bomb survivors in Japan showing an excess risk of breast cancer in younger women exposed to high doses of radiation. These data combined with the low incidence of breast cancer in young women (less than 1% of breast cancer occurs in women younger than 30 years) suggest that a restricted use of mammography is prudent. Some experts also believe that dense breast tissue, which is more common in younger women, limits the sensitivity of mammography, but studies have shown that mammography can demonstrate up to 90% of cancers in women younger than 35 years (21).
Women younger than 30 years who have a focal suspicious palpable abnormality are frequently first evaluated with US. If the US is negative and the patient is older than 25 years, a single oblique view of the affected breast may be performed to assess for suspicious microcalcifications, which would not be visualized by US. Women younger than 25 years should not undergo mammography.
If fine needle aspiration is available, it may be used in lieu of imaging studies when young patients have suspicious palpable masses. In the extremely rare circumstance of a diagnosis of carcinoma, mammography can be performed subsequently. The radiologist should be aware that a previous needle aspiration may confound the mammographic assessment of the affected area, but it will not compromise assessment of surrounding or contralateral tissues.
Increased awareness of breast cancer has led many clinicians to request more imaging studies in young women. Breast imaging cannot replace careful clinical evaluation of the breasts. If there is no suspicious focal abnormality, imaging studies will not be helpful; they may subject the patient to unnecessary risk.
Technical Considerations in Breast Imaging
Because both high-contrast and high-spatial resolution are needed for optimal mammography, standard radiographic equipment cannot be used for this examination. Mammography must be performed on a unit dedicated to this purpose. Mammographic equipment and technique differ from standard radiography in several ways. The anode material that is used to generate the x-rays in most dedicated film screen mammography units is molybdenum. This allows the production of lower energy x-rays, which in turn produces greater contrast between soft tissue structures. The structures of the breast do not differ greatly in their inherent contrast, so these low-kilovolt photons are extremely important in producing a high-contrast image. Some units also have rhodium anodes that can be used to increase the contrast in denser breasts, while keeping radiation dose and time of exposure low. Full-field digital mammography units often use tungsten anodes, which are more efficient, have better longevity, and can yield lower radiation doses than molybdenum anodes; the image processing possible with digital mammography allows high-quality mammograms utilizing tungsten anodes.
The radiologist must be able to discern tiny microcalcifications on mammograms; some of these calcifications may be 0.1 mm or less in size. The small focal spot size used in mammography units and high-resolution digital radiographic detectors or high-resolution, single intensifying screens used with single emulsion film, contribute to the creation of images with high resolution.
All mammographic units are equipped with compression paddles that squeeze the breast against the image receptor or film holder. Good compression of the breast is essential to high-quality mammography for several reasons. Compression spreads overlapping breast structures so that true masses can be differentiated from summation shadows that occur because of overlapping soft tissues. The breast is immobilized during compression so motion unsharpness or blurring due to patient movement is minimized. Geometric unsharpness, caused by the finite focal spot dimension, is minimized by bringing the breast structures closer to the film. Compression renders the breast nearly uniform in thickness so the film density of tissues near the nipple will be similar to those near the chest wall. Radiation dose can be reduced by good compression; a thinner breast requires fewer photons for penetration. Beam attenuation is also reduced.
Some women find breast compression uncomfortable, but most can tolerate it once the benefits are explained. During routine mammography the breast is compressed for a few seconds while each film is taken. Many units are equipped with automated compression devices so the technologist can release the tension immediately after the film is exposed.
Other factors are also important to consider in the production of high-quality mammograms. These include other equipment features such as type of x-ray generator, beam filtration, grid use, as well as film-intensifying screen combinations and the film-processing system. All of these factors are interrelated and must be optimized to produce technically acceptable films of the breast.
Full-Field Digital Mammography
Full-field digital mammography (FFDM) units have been commercially available since 2000 and now account for the majority of mammography units in the United States. FFDM uses an electronic system for image capture and display. It has higher contrast resolution and better dynamic range than film screen mammography. Spatial resolution is lower with FFDM, but its greater contrast resolution still makes high-quality images possible. The radiation dose from FFDM is comparable to that of film screen mammography in smaller breasts; it may be lower in larger breasts. Advantages of FFDM over film screen mammography include a higher speed of image acquisition and thus increased throughput of patients, the ability to perform image processing (which may lead to fewer repeat films due to optimization of brightness and contrast), other image-processing algorithms (which may result in increased conspicuity of certain features including microcalcifications, integration of computer-aided detection, and diagnosis software programs), electronic storage thus eliminating lost films and the need for film storage, and the possibility of teleradiology.
The Digital Mammographic Imaging Screening Trial, a multicenter trial that enrolled more than 49,000 women in the United States and Canada, found no significant differences in the sensitivity of FFDM compared to film screen mammography for all women enrolled. However, FFDM performed significantly better than film screen mammography in premenopausal and perimenopausal women, in women younger than 50 years, and in women with dense breasts (22). These findings along with the technical advantages of FFDM have resulted in the steady replacement of film screen mammography with FFDM for breast cancer detection and diagnosis.
Quality Assurance
It is the responsibility of the radiologist to assure that highest quality of breast imaging is performed at his or her facility. All standards mandated by the Mammography Quality Standards Act (MQSA) must be met. These standards apply to both film screen and FFDM. MQSA was passed into law by Congress in 1992 to ensure that all women receive optimal mammography services. The law requires that every practice become accredited by the Food and Drug Administration (FDA). Specified standards for personnel (radiologists, technologist, and physicists), equipment used, radiation dose, and quality assurance practices are stipulated. Once FDA accreditation is granted, an annual survey by a physicist must be performed to ensure that the practice continues to meet quality control and equipment standards. All facilities performing mammography are inspected annually by an FDA inspector. Each radiologist who interprets mammograms must be fully informed of the MQSA regulations. Failure to comply with the law can result in sanctions or even closure of the mammography facility.
Mammographic Positioning for Screening
Mammography can be performed with the patient seated or standing. Most screening practices prefer the standing position because it allows faster throughput and is less cumbersome. Patients are able to lean into the unit to a greater degree when standing, thus allowing more of the posterior breast tissues to be imaged. Recumbent imaging is possible, but quite difficult; its use should be restricted to problem-solving situations.
In the United States, two views of each breast are generally taken for screening mammography. In some European countries, a single mediolateral oblique (MLO) view is taken for screening examinations, but studies have shown that one-view examinations miss 20% to 25% of breast cancers (23). Moreover single-view mammography would lead to an excessive number of patients being called back for additional views. Asking large numbers of patients to return for such views would result in unacceptable levels of patient anxiety and cost. The standard views for screening mammography are the MLO view and the craniocaudal (CC) view.
 Figure 20.1. Patient Positioning for a Mediolateral Oblique View. (Courtesy of General Electric Medical Systems, Milwaukee, WI.) |
MLO View. The MLO view, when properly positioned, depicts the greatest amount of breast tissue. It is the most useful view in mammography. In countries using single-view screening, the MLO view is preferred. To perform an MLO view, the x-ray tube and image receptor, which are fixed with respect to one another, are moved to an angle that parallels the orientation of the patient’s pectoralis major muscle. The technologist is given flexibility in choosing the angle so that the greatest amount of breast tissue possible can be imaged. The angle is generally between 40° and 60° from the horizontal.
The patient is asked to relax her arm and chest muscles and to lean into the machine. The breast is placed on the image receptor and compression is applied from the superomedial direction, the same direction from which the x-rays will be generated. The breast must be pulled anteriorly and spread in a superior-inferior direction as much as possible to minimize overlapping structures and to maximize the amount of tissue imaged. The nipple should be in profile. Compression must be applied vigorously (Fig. 20.1). By convention, in the MLO view a marker indicating the side (left or right) and type of view is placed near the axillary tissues of the breast.
A properly positioned MLO mammogram should show the pectoralis major muscle down to the level of a line drawn perpendicular to the muscle through the nipple (posterior nipple line). The nipple should be in profile so that the subareolar area can be adequately evaluated. The inframammary fold should be visible to ensure that the inferior portion of the breast has been imaged (Fig. 20.2).
CC View. For the CC view, the unit is placed in the vertical position so that the x-ray tube is perpendicular to the floor. Photons will travel from the anode, located superior to the breast, to the image receptor underneath the breast. The breast is placed on the image receptor, pulled anteriorly, and spread horizontally before the compression plate is applied to the superior skin surface (Fig. 20.3). The nipple should again be in profile. The chest wall should rest against the image receptor. The markers indicating the side imaged and type of view should be placed near the skin close to the lateral aspect of the breast.
When evaluating a CC mammogram, optimal positioning can be assured when pectoralis muscle is seen centrally on the film and the nipple is in profile (Fig. 20.4). The pectoralis muscle can be visualized in about 30% of patients on the CC view. An alternative method of assuring appropriate visualization of posterior tissues is to measure the distance from the nipple to the edge of the film through the central axis of the breast; this distance should be within 1 cm of the length of the posterior nipple line as seen on the MLO view.
Interpreting the Mammogram
For interpretation, CC and MLO mammograms should each be viewed together in a mirror image configuration. This will allow the radiologist to scan the breasts for symmetry. Viewing conditions are extremely important for optimal interpretation. The room must be darkened. Computer workstations should be used for FFDM interpretation. High-resolution monitors with magnification capability are essential. If films are being interpreted, all adjacent view box light should be blocked out. Dedicated mammography film alternators and view boxes can do this automatically. If standard view boxes or alternators are used, exposed blackened film can be cut to mask out unwanted light. All visible breast parenchyma should be scanned systematically with magnification. This will allow visualization of tiny microcalcifications and will ensure that the radiologist has examined all parts of the breast in detail.
If previous mammograms are available, they should be compared to the current study so that the radiologist can evaluate the examination for any changes in the mammographic appearance of the breasts. In turn, current questionable areas can be evaluated for their stability.
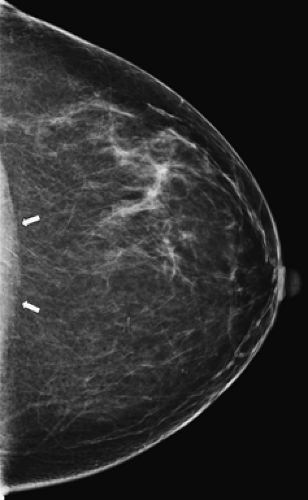 Figure 20.4. Normal Craniocaudal View of the Left Breast. Note that the nipple is in profile and the pectoralis muscle (arrows) is seen posteriorly indicating optimal visualization of breast tissue. |
In most practices, patients are asked to complete a brief history form that includes questions relevant to breast health and cancer risk. Knowledge of the patient’s history will be helpful in assessing the malignant potential and likely diagnosis of a particular mammographic finding. The risk of malignancy is much greater in a 60-year-old woman than in a 30-year-old woman. A woman with a personal or close family history of breast cancer is at greater risk for development of malignancy, and the interpretation of mammographic findings should be tailored accordingly. Other information such as previous surgical biopsies or hormone replacement intake must also be taken into account during interpretation of the mammogram.
Correlation with the physical examination is also extremely important so that false-negative reports can be minimized. All palpable lesions should be marked and assessed mammographically. Special views can image palpable lesions that occur in locations not included on standard mammography. The mammographer can also be certain that the mass felt corresponds to the mammographic abnormality. Areas of asymmetric tissue seen mammographically can be assessed for palpable abnormalities, which may render them more suspicious for malignancy.
Classic mammographic signs of malignancy are spiculated masses or pleomorphic clusters of microcalcifications; however, only about 40% of all occult breast carcinoma presents in these ways (24). In the remainder of cases, more subtle or indirect signs of malignancy are present. The radiologist must look at each mammogram with great care, utilizing all available diagnostic techniques so that false-negative diagnoses are
minimized. This charge must be balanced against the need to minimize false-positive diagnoses. Each time a woman is subjected to a surgical biopsy, financial and emotional costs as well as risks are incurred.
minimized. This charge must be balanced against the need to minimize false-positive diagnoses. Each time a woman is subjected to a surgical biopsy, financial and emotional costs as well as risks are incurred.
Table 20.2 Diagnostic Mammographic Views | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Diagnostic Evaluation of the Indeterminate Mammogram
In the majority of cases, a two-view screening mammogram will provide a conclusive interpretation, but when the results of mammography are indeterminate, further evaluation is necessary; additional mammographic views (Table 20.2) (25), US and, infrequently, MR may be required for clarification. The workup must be tailored to the specific situation.
Projections other than the standard CC and MLO views may help to visualize a lesion that is seen only in one standard view or that is obscured by surrounding parenchyma. Tangential views of the skin can be used to establish a dermal location for calcifications or superficial masses. Dermal abnormalities do not represent breast cancer.
Further characterization of an abnormality can be accomplished with spot compression and magnification views. The compression plate used is much smaller than that used in standard views; therefore, greater force can be applied, which results both in further spreading of any overlying tissue and in bringing the abnormality closer to the film for increased detail. Magnification also produces finer detail, which allows more accurate assessment of the morphology of microcalcifications and the borders of masses.
Well-defined or partially obscured masses can be evaluated with US. A high-frequency (5 to 12 MHz), hand-held linear array transducer is most commonly used. A targeted evaluation of the mammographically visible abnormality is performed. Simple cysts are easily distinguishable from complex or solid masses. This differentiation is extremely important as simple cysts are always benign and require no further workup, whereas noncystic masses may represent cancers.
MR occasionally can be used as an adjunct to mammography and sonography when there continue to be equivocal findings. MR is not, however, a replacement for more conventional imaging.
Analyzing the Mammogram
Masses
Complete assessment of a mammographically visible, potentially malignant mass requires several steps. First, the radiologist must decide whether the mass is real. The left and right breasts must be compared in each view.
Most women have reasonably symmetric parenchyma; however, at least 3% of women have areas of asymmetric, but histologically normal breast tissue. When attempting to distinguish
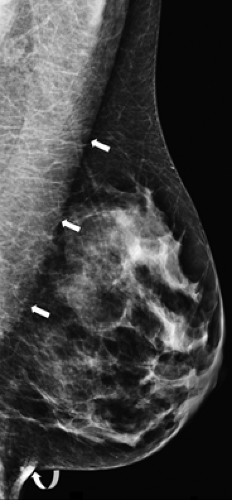
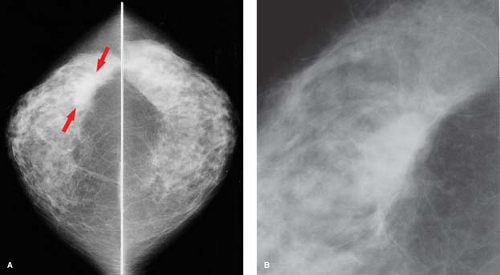
asymmetric normal breast tissue from a true abnormality, the radiologist must look for the mammographic features of a mass. Masses have convex borders and become denser toward the center. They distort the normal breast architecture. True masses are seen in multiple projections and can still be visualized when focal compression is applied (Fig. 20.5).
asymmetric normal breast tissue from a true abnormality, the radiologist must look for the mammographic features of a mass. Masses have convex borders and become denser toward the center. They distort the normal breast architecture. True masses are seen in multiple projections and can still be visualized when focal compression is applied (Fig. 20.5).
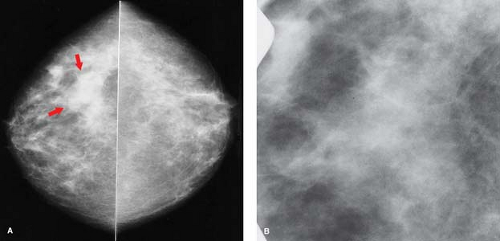
Asymmetric breast parenchyma has an amorphous quality. On spot compression, the tissue spreads apart and fat can be seen interspersed with the denser breast structures in a pattern of normal architecture (Fig. 20.6). The appearance of asymmetric tissue varies significantly from one mammographic projection to another.
When evaluating the breast for a possible mass, it is important to correlate the mammographic findings with the physical examination. When a suspicious palpable abnormality corresponds to the area of asymmetry seen on mammography, a biopsy should be undertaken. In a study of 221 patients with mammographically visible asymmetries, only 3 patients had malignancies and all 3 had suspicious, palpable abnormalities corresponding to the visualized asymmetries (26).
Summation artifacts that resemble masses on mammography can be produced by overlapping breast tissue. They are visible in only one view and usually disappear when focal compression spreads the tissues apart.
Once the radiologist has concluded that a mass is present, its margins, density, location, and size should be assessed. The number of mammographically visible masses and their similarities or differences should be analyzed. Previous films should be compared with the current study to look for new masses or an increase in the size of a mass. It is impossible to evaluate one characteristic independent of the others.
Margins
The margins of a mass are probably the most important characteristics to be assessed. Overlying breast parenchyma often obscures margin analysis, but liberal use of magnification compression views, in multiple projections, will aid the radiologist.
Spiculated Margins
Breast carcinoma classically appears as a spiculated mass on mammography (Fig. 20.7); however, less than 20% of nonpalpable cancers present as such (24). Most spiculated-appearing breast cancers will be infiltrating ductal carcinoma; however, tubular and lobular carcinomas can present as such. Tubular carcinomas are more well-differentiated histologically and carry a better prognosis. Lobular carcinomas comprise about 10% of all invasive carcinomas. They are not mammographically distinguishable from invasive ductal carcinomas, although they are frequently more subtle. Single rows of lobular cancer cells can infiltrate surrounding tissues, so they generally cause less tissue distortion.
A very limited differential exists for a spiculated mass.
Fat necrosis from a previous surgical biopsy can appear spiculated (Fig. 20.8).
Scars from previous breast surgery should be carefully marked with radiopaque wires. Comparison should be made with previous films, both to determine the location of the abnormality that underwent biopsy and to assess for any increase in size of the presumed scar. Many scars will regress with time, but others will be stable in appearance and size. Any increase in size should be viewed with suspicion and biopsy should be undertaken.
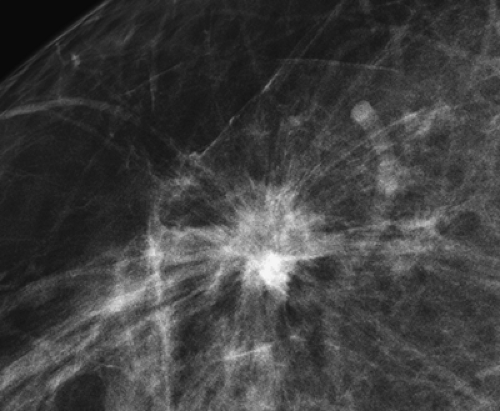 Figure 20.7. Classic Breast Carcinoma. This spiculated breast mass is an infiltrating duct carcinoma. |
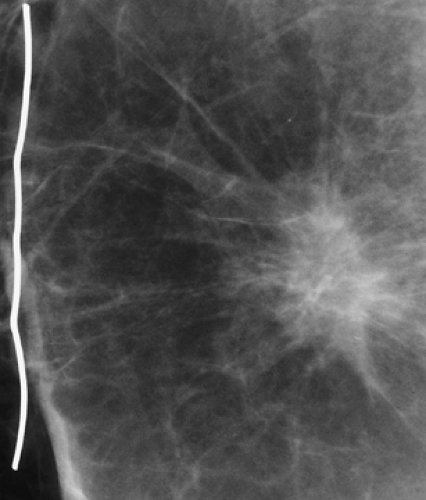 Figure 20.8. Postsurgical Fat Necrosis. This spiculated mass had been stable for 7 years. The radiopaque wire indicates the scar on the patient’s skin from the previous lumpectomy. |
Radial scar or complex sclerosing lesion can also present as a spiculated lesion. These are spontaneous lesions that are benign and consist histologically of central sclerosis and varying degrees of epithelial proliferation, represented by strands of fibrous connective tissue. Histologic differentiation of these lesions from carcinoma is mandatory.
Indistinct (Ill-Defined) Margins
Breast carcinoma can also present as a round mass with indistinct or ill-defined borders (Fig. 20.9). Benign lesions that can present as such include abscess, hematoma, and focal fibrosis.
Breast abscesses are most commonly seen in a subareolar location in lactating women (Fig. 20.10). Clinically, there is associated pain, swelling, and erythema.
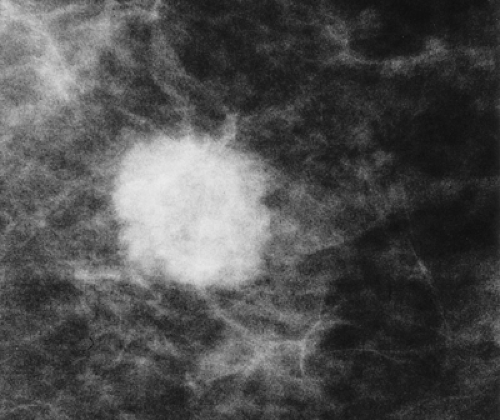 Figure 20.9. Infiltrating Duct Carcinoma. Lesion presenting as a round mass with indistinct, microlobulated borders. |
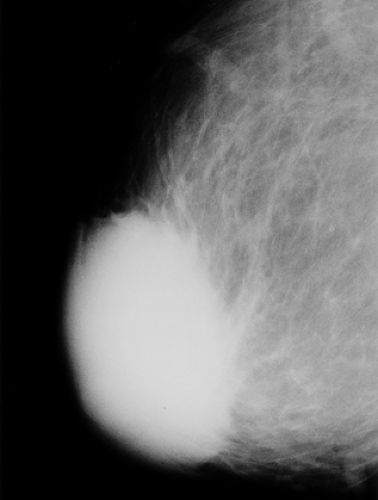 Figure 20.10. Large Subareolar Abscess. The indistinct borders of the mass are the result of surrounding inflammation. |
Spontaneous hematomas are seen in women on anticoagulant therapy or in those with blood dyscrasias. They can, of course, also be secondary to trauma, needle aspiration, or surgery. Correlation with the patient’s history and physical examination will be helpful in discerning whether a lesion represents a hematoma. If doubt persists as to the nature of a possible hematoma, short-interval follow-up mammograms (4 to 6 weeks later) to demonstrate resolution will be helpful (Fig. 20.11).
Circumscribed (Well-Defined) Margins
Circumscribed masses are almost always benign; however, up to 5% of masses that appear well circumscribed on conventional mammograms may represent carcinomas (27). The “halo sign,” which is a partial or complete radiolucent ring surrounding a mass, is not helpful in determining benignity. Sonography should be used to assess circumscribed masses prior to any additional mammographic views; if a simple cyst is diagnosed by US, no further imaging workup is required. Magnification compression views will be of great assistance in clarifying the nature of borders of an apparently well-circumscribed, solid mass. Masses that appear well circumscribed on conventional views may have indistinct or microlobulated margins on compression magnification views (28); such masses should undergo biopsy. If a solid mass appears circumscribed on magnification views and there are no previous mammograms available for comparison, the mass can generally be characterized as one that has a high probability of being benign. Such masses are frequently subjected to a course of follow-up mammography. The first of these surveillance mammograms should be performed 6 months following the original study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree