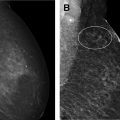
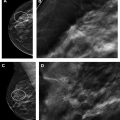
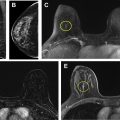
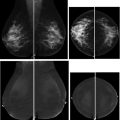
Breast magnetic resonance (MR) imaging is the most sensitive imaging modality for breast cancer detection and guidelines recommend its use, in addition to screening mammography, for high-risk women. The most recent American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) manual coordinated cross-modality BI-RADS terminology and established an outcome monitoring section that helps guide a medical imaging outcomes audit. This article provides a framework for performing a breast MR imaging audit in clinical practice, incorporating ACR BI-RADS guidance and more recently published data, clarifies common pitfalls, and discusses audit challenges related to evolving clinical practice.
Key points
- •
A medical outcomes audit is essential for assessing individual and facility-level interpretive performance.
- •
Rigorous use of the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) terminology and assessments is critical to allow accurate data capture and coding, particularly in breast magnetic resonance (MR) imaging performed for extent of disease evaluation.
- •
Breast MR imaging audit data should be captured and reported similarly to the mammography audit, with separate data calculated for screening versus diagnostic indications.
- •
Current breast MR imaging auditing guidance does not address issues related to multimodality screening use, which can result in biased estimates of test performance. Revision of auditing methods to account for multimodality screening strategies are needed to improve performance estimates in medical outcomes audits.
Background
The major goals of breast cancer screening are to find a high percentage of screen-detected cancers (cancer detection rate and sensitivity), which are more likely to be curable (minimal and early-stage, node-negative cancers), while decreasing harms such as morbidity and cost caused by additional imaging and tissue biopsies (abnormal interpretation rate and positive predictive value [PPV]). , A medical imaging outcomes audit allows for quality improvement through feedback to both individual radiologists and the entire practice compared with established benchmarks. In particular, the audit can ascertain whether individual radiologists are outside an acceptable range of performance in order to help the radiologists identify and target areas for improvement. Annual participation in the Mammography Quality Standards Act (MQSA) medical audit also fulfills part 4, Practice Quality Improvement of the American Board of Radiology (ABR) Maintenance of Certification.
The use of contrast-enhanced breast magnetic resonance (MR) imaging for early breast cancer detection has grown over the past few decades as several prospective studies showed increased sensitivity for detecting breast cancer in women with genetic or familial breast cancer predisposition. In 2007, the American Cancer Society (ACS) published guidelines for breast MR imaging screening indications. In 2010, the American College of Radiology (ACR) and Society of Breast Imaging published the breast MR imaging appropriateness criteria and the ACR established a breast MR imaging accreditation program. Unlike the required audit for mammography, the United States Food and Drug Administration currently does not have regulations that mandate a medical outcomes audit of a breast MR imaging practice. However, a breast MR imaging outcomes audit, which includes interpretation accuracy and appropriate clinical indication, is required for practice accreditation through the ACR breast MR imaging accreditation program.
The most recent 2013 edition of the ACR Breast Imaging Reporting and Data System (BI-RADS) atlas aligned cross-modality BI-RADS terminology and created a follow-up and outcome and monitoring section focusing on the medical audit. Breast MR imaging screening benchmarks were based on analysis of prospective screening MR imaging trials of women at high risk for breast cancer, because large-scale breast MR imaging performance data from the United States clinical practice had yet to be published. Since then, additional research has been performed to improve performance measures for both screening and diagnostic breast MR imaging. , This article provides a framework for performing a breast MR imaging audit in clinical practice incorporating ACR BI-RADS guidance and more recent data, clarifies common pitfalls, and discusses audit challenges related to evolving clinical practice.
Overview of the breast magnetic resonance imaging audit
Similar to the more established mammography audit, the breast MR imaging audit involves gathering and analyzing data from several sources: the breast MR imaging report, subsequent imaging and biopsy recommendations, and breast biopsy and surgical pathology. To help with data analysis, a standardized reporting system for reporting and coding should be followed, and appropriate use of the ACR BI-RADS lexicon when interpreting breast MR imaging examinations is critical. It is also essential to have an organized system for correlation of biopsy results with positive MR imaging studies for outcomes tracking.
The MR imaging audit is based on the overall BI-RADS assessment at the examination level, according to the most actionable BI-RADS lesion category, with recommendations for core biopsy (BI-RADS category 4 or 5) superseding that of a biopsy-proven malignancy (BI-RADS category 6). Institutions may choose to report individual findings at the lesion level and/or breast level to facilitate coordination and communication of patient care. However, breast MR imaging studies with a BI-RADS category 6 assessment at the examination level should not be included in the medical audit because this would skew certain performance parameters.
An audit should be calculated once every screening interval. In general, this is assumed to be every year, because this is the most common screening interval in the United States; however, if a facility uses a longer screening interval, such as 2 years, this length of time should be substituted. The audit should include data moving forward for at least 1 year to allow sufficient time for diagnostic procedures, pathology results, and outcomes data capture to produce meaningful audit outcomes.
Data collection and definitions
The ACR BI-RADS atlas includes guidelines for data collection for both the basic clinically relevant audit and the more complete audit ( Box 1 ). A basic clinically relevant audit provides a minimum amount of data to assess interpretive performance. These data include the number of screening and diagnostic examinations performed over a period of time and ACR BI-RADS category 0, 3, 4, and 5 recommendations. Subsequent tissue diagnosis pathology results and minimal cancer staging information are also needed. Recommendations for performance metrics to be calculated for the basic clinically relevant audit for breast MR imaging are similar to those from a mammography audit and include cancer detection rate (CDR), sensitivity, specificity, positive predictive values for biopsy recommendation and for biopsies performed, as well as characteristics of cancers: median size of invasive cancers, percentages of minimal cancer, stage 0 or 1 cancers, and node-negative invasive cancers.
- 1.
Modality or modalities.
- 2.
Dates of audit period and total number of examinations in that period (usually a 12-month period).
- 3.
Risk factors:
- •
Patient’s age at the time of the examination.
- •
Breast and ovarian cancer history: personal or family (especially premenopausal breast cancer in first-degree relative—mother, sister, or daughter).
- •
Previous biopsy-proven hyperplasia with cellular atypia, or lobular carcinoma in situ (LCIS).
- •
Hormone replacement therapy.
- •
Breast density as estimated at mammography.
- •
- 4.
Number and type of examination: screening (asymptomatic), diagnostic (evaluation of clinical symptoms or signs suggesting the possibility of breast cancer, evaluation of screening-detected findings, short-interval follow-up examinations). a
- 5.
First-time examination or not.
- 6.
Number of recommendations for:
- •
Additional imaging evaluation (recall) (BI-RADS category 0 = “Need Additional Imaging Evaluation”).
- •
Routine (usually annual) screening (BI-RADS category 1 = “Negative” and category 2 = “Benign”).
- •
Short-interval follow-up (BI-RADS category 3 = “Probably Benign”).
- •
Tissue diagnosis (BI-RADS category 4 = “Suspicious” and category 5 = “Highly Suggestive of Malignancy”).
- •
- 7.
Tissue diagnosis results: malignant or benign, for all ACR BI-RADS category 0, 3, 4 and 5 assessments (keep separate data for fine-needle aspiration, core biopsy and surgical biopsy cases). MQSA Final Rule requires that an attempt is made to collect tissue diagnosis results only for mammography examinations for which tissue diagnosis is recommended.
- 8.
Cancer data:
- •
Imaging findings: mass, calcifications, other signs of malignancy (including architectural distortion and the several types of asymmetry), no signs of malignancy.
- •
Palpable or nonpalpable at time of imaging examination
- •
Cancer staging: histologic type, invasive cancer size, nodal status, and tumor grade.
- •
- 9.
MQSA Final Rule also requires analysis of any known false-negative mammography examinations by attempting to obtain surgical and/or pathology results and by review of negative mammography examinations.
Note: Bolded items indicate data included in the basic clinically relevant mammography audit.
a Separate audit statistics should be maintained for screening examinations and for each of the subtypes of diagnostic examinations.
The ACR also encourages collecting additional data to perform a more complete audit, which can provide additional important performance information. This audit includes collecting data on history of prior breast MR imaging examinations (for prevalent vs incident performance metrics), patient breast cancer risk factors (indications for breast MR imaging), mammographic breast density, type of imaging findings, and whether or not the cancer was palpable at the time of imaging. The more complete audit also includes sensitivity and specificity performance calculations, for which linkage to a pathology database or regional cancer registry will be needed to capture these data. Facilities without linkage to these databases have difficulties capturing both true-positive and true-negative examinations for these calculations.
Data Definitions
The following data definitions are defined by the ACR BI-RADS atlas.
Screening examination: performed in asymptomatic women for early detection of clinically unsuspected breast cancer. For breast MR imaging, this includes surveillance examinations for asymptomatic women with a personal history of breast cancer and asymptomatic women with history of breast reconstruction.
Diagnostic examination: breast MR imaging performed for extent of disease for women with recent diagnosis of breast cancer before receipt of definitive treatment, patients with clinical signs or symptoms that may suggest breast cancer, problem solving (abnormal mammogram or ultrasonography examination), follow-up of a prior BI-RADS category 3 finding, or follow-up for neoadjuvant chemotherapy to assess for treatment response. Although the BI-RADS atlas indicates that diagnostic breast MR imaging may be performed for short-interval follow-up after breast conservation therapy, if the woman is asymptomatic, then classifying the MR imaging as a screening examination most closely aligns with the intent of the medical outcomes audit. Of additional note, examinations with BI-RADS category 6 assessments should not be included in the audit, because the purpose of the audit is to evaluate performance for breast cancer detection.
Positive examination: the images acquired for a breast MR imaging examination are the same regardless of whether the indication is screening or diagnostic. However, for auditing purposes, the positivity criterion varies with indication. For screening MR imaging examinations, BI-RADS category 0, 3, 4, or 5 results are considered positive, where additional evaluation with either imaging or tissue biopsy are recommended. The use of BI-RADS category 0 (additional imaging) on MR imaging should be an infrequent occurrence and used only if the images are technically inadequate or if there a different imaging modality is recommended to confirm a benign imaging findings (such as a lymph node on ultrasonography). For diagnostic MR imaging examinations, only BI-RADS category 4 or 5 results are considered positive where tissue diagnosis via biopsy is recommended.
Negative screening or diagnostic examination: this is when no tissue diagnosis is recommended. For screening MR imaging, only routine follow-up is recommended (BI-RADS category 1 or 2). For diagnostic MR imaging, BI-RADS category 1, 2, or 3 assessments are considered negative, because no tissue diagnosis is recommended. For BI-RADS category 3 assessments, when used for the first time on a screening breast MR imaging examination, the category 3 assessment is a positive screening result, because additional imaging is recommended; however, on subsequent short-interval follow-up diagnostic MR imaging, it would be coded as a negative diagnostic result given that a tissue diagnosis is not recommended.
Tissue diagnosis: this includes any pathologic diagnosis after an interventional procedure such as fine-needle aspiration, core needle biopsy, or excisional biopsy. A tissue diagnosis includes cases where a diagnostic aspiration was performed to differentiate between a complicated cyst versus a solid mass, even if the fluid obtained was not sent for cytologic analysis.
Cancer: a tissue diagnosis of breast cancer (ductal carcinoma in situ or invasive breast cancer) within the recommended screening interval after the imaging examination. Pathology diagnosis of high-risk lesions such as atypical ductal carcinoma, lobular neoplasia, or nonbreast cancers (sarcoma, lymphoma, metastasis to the breast) are not classified as true-positive or a cancer in the medical outcomes audit.
Comparison with benchmarks
The medical outcomes audit is most useful for comparisons with standard benchmarks representative of the patient population. At the time of publication of the fifth edition of the ACR BI-RADS manual, the screening MR imaging benchmarks were based on prospective clinical trials of women at high risk of breast cancer, because there were insufficient breast MR imaging audit data from United States clinical practices. , , Since 2013, additional studies have narrowed this knowledge gap.
The largest screening breast MR imaging outcomes study used data from the National Cancer Institute–funded Breast Cancer Surveillance Consortium (BCSC), which includes a geographically and ethnically diverse sample of the United States population and breast imaging data from facilities linked to outcome data from state and regional tumor registries. This study found that most performance parameters met or approached the ACR BI-RADS benchmarks ( Table 1 ), and also provided additional values for 2 benchmarks categorized as to be determined in the BI-RADS atlas: median size of invasive cancers and percentage of stage 0 or 1 cancer. Multi-institutional diagnostic breast MR imaging outcomes data have yet to be published, although screening and diagnostic breast MR imaging examinations should be audited separately because there is significant difference in abnormal interpretation rates and other metrics based on clinical indication. ,